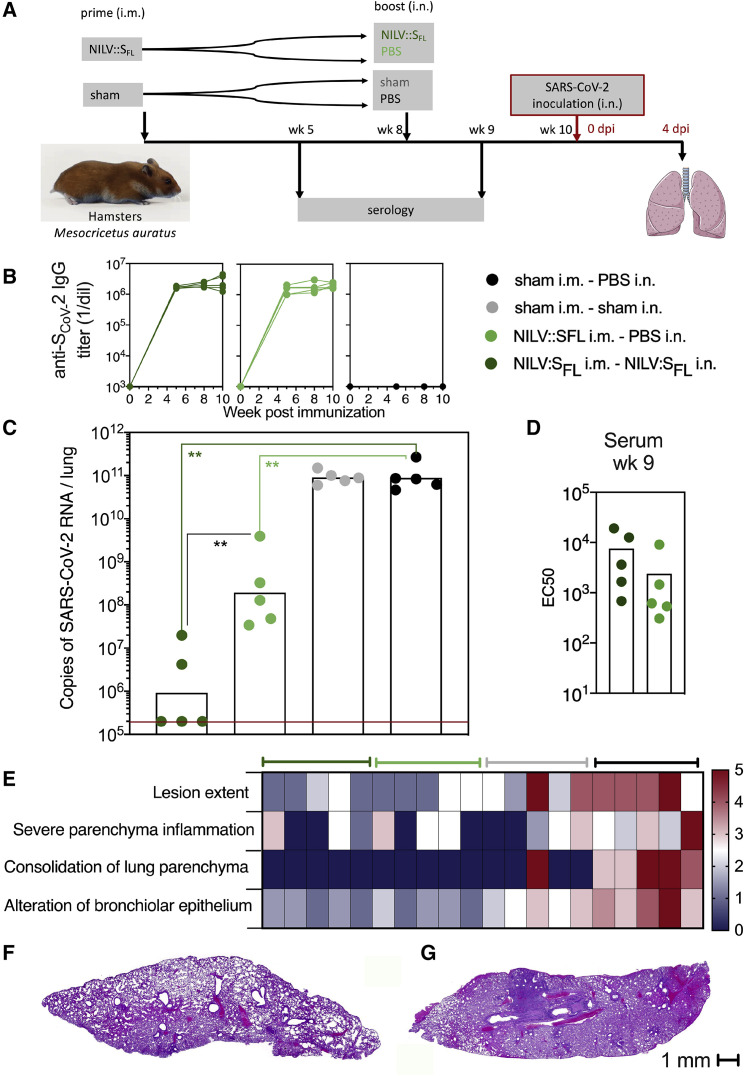
Figure 7.
Protective efficacy of NILV::SFL in a systemic prime and i.n. boost regimen in hamsters
(A) Timeline of the NILV::SFL prime-boost or prime-target immunization regimen and challenge.
(B) Profile of serum anti-SCoV-2 IgG response following a single (i.m.) injection or a prime (i.m.)-boost (i.n.) immunization with NILV::SFL.
(C) Lung viral loads at 4 dpi with SARS-CoV-2 in controls or NILV::SFL-vaccinated hamsters. Statistical significance was evaluated by a two-tailed unpaired t test; ∗∗p < 0.01.
(D) EC50 serum neutralizing titers after the boost-target regimen.
(E) Lung histological H&E analysis shown in a heatmap recapitulating the histological scores for each parameter defined in Figure S7C and determined for individuals of various groups at 4 dpi.
(F and G) Representative whole-lung section from NILV::SFL i.m.-NILV::SFL i.n. (F) or sham i.m.-sham i.n. (G) hamsters. See also Figure S7.