Abstract
Acute physical or psychological stress can elicit adaptive behaviors that allow an organism maintain homeostasis. However, intense and/or prolonged stressors often have the opposite effect, resulting in maladaptive behaviors and curbing goal-directed action; in the extreme, this may contribute to the development of psychiatric conditions like generalized anxiety disorder, major depressive disorder, or post-traumatic stress disorder. While treatment of these disorders generally focuses on reducing reactivity to potentially threatening stimuli, there are in fact impairments across multiple domains including valence, arousal, and cognition. Here, we use the genetically stress-susceptible 129S1 mouse strain to explore the effects of stress across multiple domains. We find that 129S1 mice exhibit a potentiated neuroendocrine response across many environments and paradigms, and that this is associated with reduced exploration, neophobia, decreased novelty- and reward-seeking, and spatial learning and memory impairments. Taken together, our results suggest that the 129S1 strain may provide a useful model for elucidating mechanisms underlying myriad aspects of stress-linked psychiatric disorders as well as potential treatments that may ameliorate symptoms.
Keywords: Stress, Genetic background, Glucocorticoids, Corticosterone, Anhedonia, Motivation, Learning, Memory
Abbreviations: HPA, Hypothalamic-pituitary-adrenal; CORT, corticosterone; PTSD, post-traumatic stress disorder; GR, glucocorticoid Receptor; RDoC, Research Domain Criteria; FED, Feeding Experimentation Device 3; FR, frequency-ratio 1; PR, progressive-ratio
1. Introduction
The experience of stress triggers the activation of multiple physiological and behavioral coping mechanisms that promote survival. Collectively, these changes are part of a dynamic process termed allostasis, which equips organisms to adapt in disparate stressful environments (McEwen and Akil, 2020; McEwen and Gianaros, 2011). The allostatic response recruits widespread physiological functions to return the organism to homeostasis. For example, activation of the hypothalamic-pituitary-adrenal axis (HPA) leads to synthesis and release of glucocorticoids, such as cortisol in humans or corticosterone in rodents (CORT), which impact energy balance, glucose utilization, brain function and behavior (Spencer and Deak, 2017). Importantly, the actions of CORT in the brain are also essential for terminating the HPA axis stress response through a negative-feedback loop; for example, CORT binds to glucocorticoid receptors (GR) in the hippocampus, which in turn inhibit HPA activation via inputs to the paraventricular nucleus in the hypothalamus (Cullinan et al., 1993; Herman et al., 1995).
However, individuals exhibit considerable variability in their sensitivity (i.e. the threshold for activating allostasis) as well as the reactivity to stressors (i.e. the extent of an allostatic response). Many studies suggest that individual variation in stress susceptibility strongly modulates the propensity for developing psychopathologies such as generalized anxiety disorder, major depressive disorder, and post-traumatic stress disorder (PTSD) (Daviu et al., 2019; Holmes and Singewald, 2013; Pizzagalli, 2014). Insights into the risks posed by a dysregulated stress response can be gained from studying innately susceptible populations (in humans (McLean et al., 2019; Vasterling et al., 2002) and in mice (Krishnan et al., 2007; Lebow et al., 2012; Razzoli et al., 2010). In mice, comparisons between isogenic strains differing in stress sensitivity and reactivity have been used to further our understanding of maladaptive responses to stress (Moore et al., 2020). Mice from the 129S1/SvImJ strain (129S1) are considered a stress-susceptible population because they exhibit dysregulated HPA axis function relative to other strains, such as the C57BL/6 (Camp et al., 2012). For example, 129S1 mice exhibit greater serum corticosterone (CORT) concentrations in response to acute restraint stress (Camp et al., 2012). In addition, a low dose injection of the synthetic glucocorticoid, dexamethasone, fails to suppress further CORT release suggesting failure of the negative feedback system (Camp et al., 2012). Consistent with this, 129S1 mice also exhibit genetic differences, including reduced GR mRNA expression in the CA3 region of the hippocampus and reduced expression of Ppid in the amygdala (Gunduz-Cinar et al., 2018). Interestingly, the family containing Ppid (tetratricopeptide repeat [TPR] proteins) also includes FKBP5, which is one of the most common genetic markers for susceptibility to anxiety and trauma-related disorders in human populations (Appel et al., 2011; Binder et al., 2008; Le-Niculescu et al., 2019). Altogether, these findings indicate that a dysregulated HPA axis may contribute to higher stress sensitivity and reactivity in 129S1 mice.
Accompanying a dysregulation of the HPA axis in 129S1 mice are impairments in fear learning and memory, which have been studied using Pavlovian conditioning and extinction paradigms (Camp et al., 2009; Hefner et al., 2008). In fear conditioning, rodents learn to respond defensively to a conditioned stimulus, which has been repeatedly paired with an aversive outcome (for example, defensive behavior like freezing is displayed in response to a conditioned auditory stimulus that has been paired with an aversive mild footshock). After learning this association, fear extinction learning can be engaged by exposing rodents to the conditioned stimulus without the aversive outcome; they progressively learn to reduce their defensive response to the conditioned stimulus since it no longer is predictive of the aversive outcome. Interestingly, there are no differences between 129S1 mice and other stains in fear conditioning (Fitzgerald et al., 2014), but they exhibit severe deficits in their ability to adaptively reduce their defensive behavior. For example, relative to C57BL/6 mice, 129S1 mice exhibit persistent defensive behavior after extinction training (Camp et al., 2009; Hefner et al., 2008), a greater degree of generalization (displaying defensive behavior when stimuli that are different than, but similar to, the conditioned stimulus are presented (Camp et al., 2009; Cazares et al., 2019; Temme et al., 2014); and deficiencies in discriminating between stimuli signaling aversive and “safe” outcomes (Camp et al., 2012). Importantly, fear learning deficits are not ameliorated when 129S1 mice are fostered by C57BL/6 parents prenatally, postnatally, or after weaning (Gunduz-Cinar et al., 2018). Altogether, these findings suggest that 129S1 mice exhibit a dysregulated physiological response to stress that leads to severe associative fear learning deficits.
Thus far, studies of 129S1 mice have focused almost exclusively on anxiety-related behaviors and aversively motivated, associative learning tasks, such as Pavlovian fear conditioning and fear extinction. However, it is well established that stress affects multiple behavioral domains and alters widespread neural function (Daviu et al., 2019; Lopez and Flagel, 2020; Quervain et al., 2016). Our goal was to investigate behavioral performance across multiple domains in the stress-susceptible 129S1 strain. We found that, relative to C57BL/6 mice, 129S1 mice exhibited higher CORT levels (even in a lower-stress home-cage environment), decreased exploration using intrinsically motivated tasks (like novel tactile-object recognition or social recognition), decreased operant responding for reward, and impairments in spatial learning and memory.
Our results suggest that increased stress reactivity is associated with a myriad of behavioral pathologies in 129S1 mice. These phenotypes are aligned with the complex, multifaceted traits that characterize human psychopathology (Insel and Cuthbert, 2009). Therefore, the stress-susceptible 129S1 strain may represent a model to elucidate distinct and overlapping mechanisms underlying maladaptive behaviors along multiple Research Domain Criteria (RDoC), including positive and negative valence, arousal, and cognition (Sanislow et al., 2019).
2. Materials and methods
2.1. Animals
Male and female mice were obtained from commercial vendors or bred in our colony using naive mice from the same vendors. The C57BL/6NTac mice were obtained from Taconic Farms (Model #B6NTac; Hudson, New York City) and the 129S1/SvImJ mice obtained from Jackson Laboratories (Stock # 002448; Bar Harbor, Maine); hereafter these are referred to as C57BL/6 and 129S1, respectively. C57BL/6 mice were chosen as the comparison strain in this study for two reasons: 1. C57BL/6 represent one of the most commonly used mouse lines in neuroscience research, and 2. Previous work evaluating fear learning in the 129 strains typically use C57BL/6 as a reference, since they exhibit good fear extinction acquisition and recall (Camp et al, 2009, 2012). Mice were housed by sex (maximum 5 per cage) in a temperature-controlled vivarium (22 °C) with a 14-h/10-h light/dark cycle for a minimum of 4 weeks prior to studies. Mice were provided with ad libitum food and water, except where specified during operant learning experiments. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan and in accordance with guidelines for animal care set by the National Institute of Health.
Experiments were conducted using age-matched mice with approximately equal number of males and females in each cohort; details for each cohort (including sex distribution, range and average age, and experiments performed) are presented in Supplemental Table 1. Sample sizes were based on power calculations, designed to detect a minimum effect size of 0.2 with 80% power and an alpha value of 0.05.
2.2. Open-field exploration
To assess differences in exploration levels between strains, open-field experiments were conducted as described previously (Krueger et al., 2016), using Cohort 1 (n: B6 = 47 [27M, 20F]; S1 = 70 [52M, 18F]; Fig. 1). Briefly, experiments were carried out in a rectangular (53 x 38 × 26 cm) or round (45 cm diameter) open arena composed of smooth white opaque acrylic walls and floor (Chemtainer, Lombard, IL). Illumination of the arena, measured in the center, was ~45 lx. Immediately prior to open-field experiments, each cage of mice was group-habituated (all mice from the same cage placed into one arena together) for 10 min. The open-field experiment consisted of three successive trials in one day. In each trial, mice were individually placed in the center of an arena and allowed to freely explore for 10 min. Between each trial, mice were placed in individual clean holding cages for ~5–10 min while the open field arenas were cleaned with 70% ethanol. LimeLight 3 video tracking software (Actimetrics, Evanston, IL), run on a desktop PC, and individual cameras mounted above each open-field arena were used to record behavior and analyze total distance traveled and time spent in the inner zone of the arena (24 × 15 cm for rectangular; 27 cm diameter for circular).
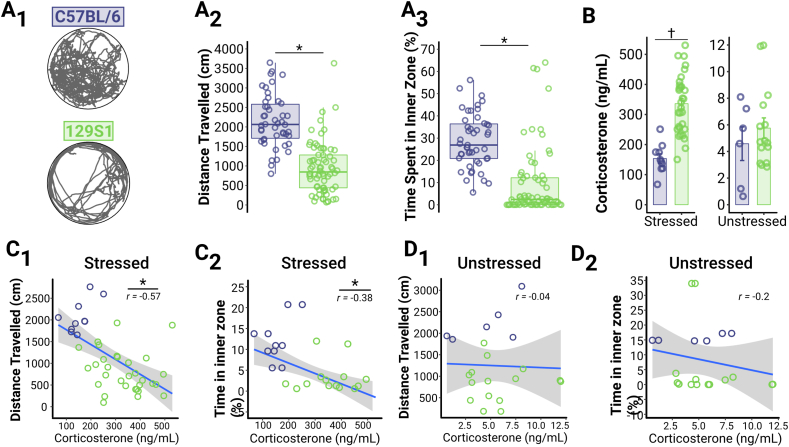
Fig. 1.
129S1 mice exhibit reduced exploration in an open field, which is associated with higher CORT levels.
(A) Comparison between C57BL/6 (n = 47 [27M, 20F]) and 129S1 (n = 70 [52M, 18F]) mice (comprising Cohort 1) in open field exploration. (A1) Representative trajectories of individual animals in an open field during a 10-min trial. (A2-A3) 129S1 mice traveled a shorter distance (A2, p < 0.0001) and spent less time in the inner zone (A3, p < 0.0001) in the open field compared to C57BL/6 mice. Boxplots (A2-A3) show the range (whiskers) and the median (line) as well as 25th and 75th quartiles (box edges). (B) Stress-reactivity was compared in a subset of mice from Cohort 1 by measuring plasma CORT levels after 30 min of restraint stress (“Stressed”; C57BL/6, n = 10M; 129S1, n = 31M) and in mice not receiving restraint stress (“Unstressed”; C57BL/6: n = 6M; 129S1: n = 15M). Within the Stressed group, 129S1 mice exhibited higher CORT levels than C57BL/6 mice (p < 0.0001), but there were no differences in the Unstressed group (p = 0.9735). (C) CORT levels in the Stressed group were inversely related to distance traveled (C1, p < 0.0001) and to percent time spent in the inner zone (C2, p = 0.0146) in the open field. (D) CORT levels from Unstressed mice showed no relationship to distance traveled (D1, p = 0.8726) or to percent time spent in the inner zone (D2, p = 0.3845). For all panels, asterisks indicate significant main effects and crosses indicate significant post-hoc tests. For C-D, linear fits are displayed ± SEM and Pearson's r is shown on the graphs.
2.3. Novel tactile-object recognition
Novel tactile-object recognition was performed as previously described (Watson et al., 2020), using the same cohort (Cohort 1; Fig. 2A,C,E) as the open-field exploration trials (above). Open-field exploration trials completed on Day 1 served as the habituation trials for the Novel tactile-object recognition experiment. On Day 2 (familiarization phase), mice were individually allowed 10 min to explore two identical sandpaper grits (60 or 100) adhered to large binder clips placed on opposite sides in the center of the arena. During a short inter-trial interval (5–10 min), mice were placed in clean holding cages and binder clips were replaced, one each with the 60 or 100 grit sandpaper, providing a ‘familiar’ and ‘novel’ grit. Mice were then returned to the arena and allowed to explore for another 10 min (recognition phase). Between each trial and each animal, the arena and binder clips were cleaned with 70% ethanol. LimeLight 3 video tracking software (Actimetrics, Evanston, IL), run on a desktop PC, and individual cameras mounted above each arena were used to record behavior. XY coordinate data (in pixel units) was extracted using the open source software, DeepLabCut (Mathis et al., 2018). Subsequently, data was imported to R Studio (https://rstudio.com/) for graphing and statistical analyses using open-source packages (e.g. Nuñez et al., 2018; Wickham et al., 2019). Mice were deemed to be interacting with an object if the animal's nose was within a small zone (1 cm perimeter) surrounding the object for a minimum of 1 s. Previous studies have used time spent in an interaction zone as a measure of exploration (Krishnan et al., 2007; Moore et al., 2013); however, this measure was confounded for analysis of 129S1 mice as they often remain immobile within the interaction zone for the duration of an experiment. Therefore, we opted to use alternations (sequential interaction with each object) as a more accurate index of exploration.
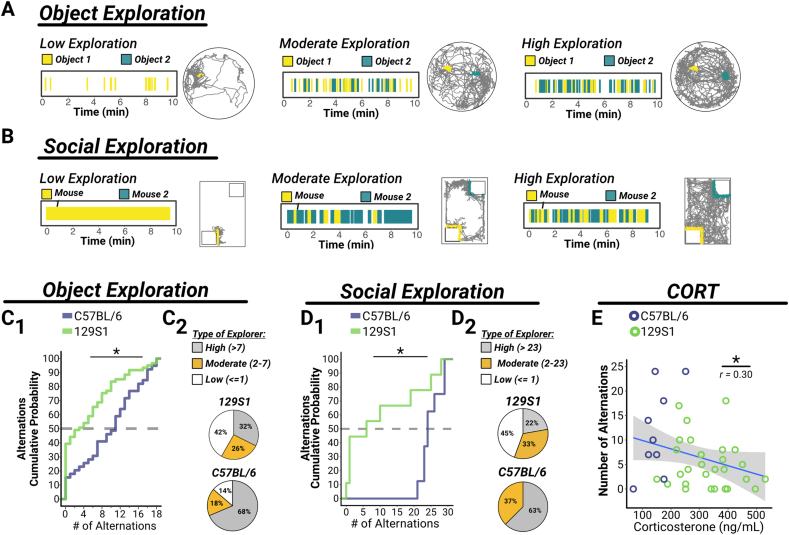
Fig. 2.
129S1 mice exhibit reduced object and social exploration, which is associated with higher CORT levels.
(A–B) Representative examples of raster plots (depicting interactions) and trajectory maps during object (A; Cohort 1) and social (B; Cohort 2) exploration during a 10-min trial. (C–D) Quantification of alternations as a measure of exploration shows that 129S1 mice (object: n = 70 [52M, 18F]; social: n = 9 [5M, 4F]) explore significantly less than C57BL/6 mice (object: n = 47 [27M, 20F]; social: n = 8 [4M, 4F]) in both object (C1, p < 0.0001) and social (D1, p = 0.0119) paradigms. Additionally, the proportions of high, moderate, and low explorers (based on the median number of alternations for all mice) in both object (C2) and social (D2) exploration differed between the strains, where a plurality of 129S1 mice were low explorers and a majority of C57BL/6 mice were high explorers. (E) There was a significant inverse relationship between CORT levels measured after 30 min of restraint stress (from a subset of Cohort 1 mice: C57BL/6, n = 10M; 129S1, n = 31M) and the number of alternations during object exploration (p = 0.0153). Linear fit is displayed ± SEM and Pearson's r is shown on the graph. For all panels, asterisks indicate significant main effects.
2.4. Social recognition
Social recognition experiments were conducted as previously described (Zhao et al., 2018), using mice in Cohort 2 (n: B6 = 8 [4M, 4F]; S1 = 9 [5M, 4F]; Fig. 2B,D). Briefly, two holding chambers (10 x 10 × 10.5 cm) were placed in opposite corners of a rectangular arena (53 x 38 × 26 cm). The holding chambers were covered with wire mesh to allow for odor cues and limited physical interaction between the sex-matched stimulus mice (in the chambers) and the experimental animal. Prior to the beginning of the experiment, the stimulus mice (age, sex, and strain-matched to the experimental mice) were habituated to the holding chambers for two 20-min periods. On Day 1, experimental mice were habituated to the empty arena (no chambers or stimulus mice present) by being allowed to explore for 10 min. On Day 2, during the familiarization phase, the arena contained one chamber housing a stimulus mouse (mouse A) and one empty chamber; experimental mice were again allowed to explore for 10 min. During a short inter-trial interval (5–10 min), the experimental mouse was placed in a clean holding cage and a second stimulus mouse (mouse B) was introduced into the empty chamber (mouse A remained in the original chamber). Experimental mice were returned to the arena and allowed to explore for 10 min (recognition phase). Each arena was cleaned with 70% ethanol between trials with different experimental mice. A third day of the experiment was conducted (in which the experimental mouse was exposed to a familiar stimulus mouse from Day 2 and a new, unfamiliar mouse) with the goal of assessing long-term memory; however, a lack of exploration of the stimulus mice on Day 2 precluded the ability to accurately analyze this data. Behavior was recorded (with LimeLight 3 software) and exploration analyzed (with DeepLabCut and R studio software) as in the Novel Tactile-Object Recognition experiments described above.
2.5. Operant learning
Appetitively motivated operant learning was assessed using the Feeding Experimentation Device 3 (FED), as described (Nguyen et al., 2016). FEDs can be used for automated feeding or to train mice to nose-poke for food pellets with little-to-no experimenter intervention over long periods of time (hours to days), which can reduce stress and novelty-induced suppression of operant responding. FED tasks were carried-out in a modified home-cage environment, in which mice were singly housed and water was available ad libitum, but food could only be obtained in 20 mg pellets from the FEDs (up to two FEDs were mounted per cage, depending on the specific experiment). Each nose-poke and pellet retrieval event for a 24-hr period was automatically logged by each individual FED and stored in CSV format on an 8 GB SD card. FED tasks analyzed for distinct cohorts are described in more detail below.
Pellet Preference and Progressive Ratio. For pellet preference, mice (Cohort 3; n: B6 = 12 [5M, 7F]; S1 = 70 [6M, 6F]; Fig. 3A,B,D) were exposed to two FEDs positioned on opposite ends of the cage. Each FED contained a different pellet type, either standard chow (5TUM, 20 mg, TestDiet) or sweetened chocolate (Dustless Precision Pellets®, 20 mg, Rodent Purified Diet). During this task, the FEDs dispensed a pellet each time a pellet was retrieved from the food receptacle (no nose-poke was required for pellet delivery). Pellet preference was conducted for three consecutive 24-h sessions. A preference ratio was calculated for each mouse (chow pellets – chocolate pellets/chow pellets + chocolate pellets). Progressive ratio experiments were then used to evaluate strain differences in motivation by measuring the extent to which mice maintained operant responding (nose-poking) under increasing work demands (Hailwood et al., 2018). After mice reached a minimum of 65% correct nose-pokes on a fixed ratio 1 (FR1) schedule (which required 1–2 days of training; data not shown), they were shifted to a progressive ratio (PR) schedule where the number of nose-pokes required to receive a pellet increased by the following formula: [(5 * e (pellet number *0.2)) – 5] (Richardson and Roberts, 2004). The total number of nose-pokes performed over a 24-hr period and the breakpoint, defined as the nose-pokes-to-pellet ratio at which the animals ceased operant responding for >2 h, were evaluated for each mouse.
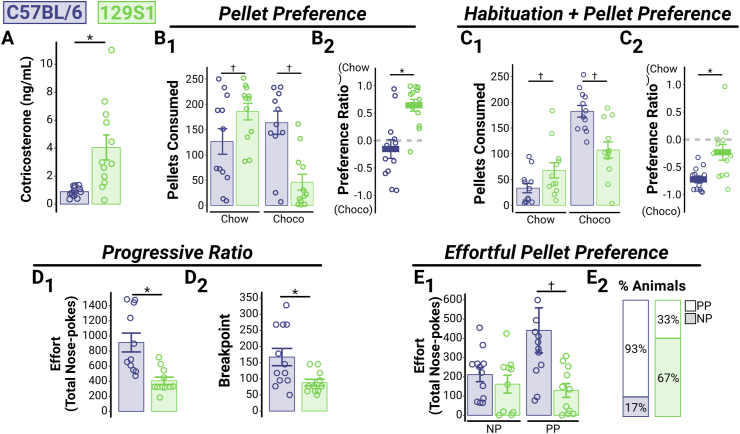
Fig. 3.
129S1 mice exhibit higher CORT levels and reduced reward-seeking, even in a lower-stress environment.
(A) CORT levels, measured at the conclusion of operant behavioral tasks, were higher in 129S1 mice (n = 12 [6M, 6F]) compared to C57BL/6 mice (n = 12 [5M, 7F]) (together, comprising Cohort 3), even in a home-cage environment (p = 0.002). (B) In the same cohort (Cohort 3), evaluation of pellet preference was carried out using standard chow and sweetened chocolate pellets; 129S1 mice consumed more chow pellets (B1 “Chow”, p = 0.049) and fewer chocolate pellets (B1 “Choco”, p = 0.0003) than C57BL/6 mice over a 24-h period (average of three consecutive days). Further, preference ratios calculated for individual animals showed that 129S1 mice exhibited a significantly stronger preference for the chow pellets than the C57BL/6 mice (B2, p = 0.001). The dashed line indicates an equal number of chow and chocolate pellets eaten (preference ratio of 0). (C) To account for the possibility of neophobia to the chocolate pellets, a separate cohort (Cohort 4) of C57BL/6 mice (n = 12 [7M, 5F]) and 129S1 mice (n = 12 [6M, 6F]) received three days of habituation to both pellet types prior to beginning the pellet preference experiment. Even after habituation, 129S1 mice still consumed more chow pellets (C1 “Chow”, p = 0.0499) and fewer chocolate pellets (C1 “Choco”, p = 0.0002) than the C57BL/6 mice. While the relative preference for chocolate pellets increased in both strains (compare to B1), C57BL/6 mice still exhibited a significantly stronger preference ratio for chocolate pellets than did the 129S1 mice (C2, p = 0.004). The dashed line indicates an equal number of chow and chocolate pellets eaten (preference ratio of 0). (D) Motivation to work for a food reward was assessed using a progressive ratio reinforcement schedule (in Cohort 3 mice). 129S1 mice expended less effort (fewer total nose-pokes; D1, p = 0.001) and had a lower breakpoint (D2, p = 0.014) than did C57BL/6 mice. (E) In a separate cohort (Cohort 5; C57BL/6: n = 12 [6M, 6F]; 129S1: n = 10 [6M, 4F]), we assessed the relative valuation of food rewards by comparing performance when a high-cost/high-reward (progressive ratio and preferred pellet [PP]) and a lower-cost/lower-reward (fixed-ratio 5 and non-preferred pellet [NP]) option were both available. 129S1 mice expended equal effort for the lower-cost/lower-reward option (E1 “NP”, p = 0.6095), but significantly less effort for the high-cost/high-reward option (E1 “PP”, p = 0.0037) compared to the C57BL/6 mice. Additionally, we found that the majority of 129S1 mice spent most of their effort on the lower-cost/lower-reward choice while only a small fraction of C57BL/6 mice did the same (E2). For all panels, asterisks indicate significant main effects and crosses indicate significant post-hoc tests.
Habituation + Pellet Preference. To ensure that neophobia to the pellets did not confound the interpretation of strain differences in pellet preference, a separate cohort of mice (Cohort 4; n: B6 = 12 [7M, 5F]; S1 = 12 [6M, 6F]; Fig. 3C) were evaluated in pellet preference (as above) after an extended habituation period. The habituation period consisted of 3 days of ad libitum access to both pellets prior to introduction of the FEDs.
Effortful Pellet Preference. In another cohort of mice (Cohort 5; n: B6 = 12 [6M, 6F]; S1 = 10 [6M, 4F]; Fig. 3E), after pellet preference and FR1 (data not shown), we evaluated the relative incentive value for each animal's preferred pellet type. Mice were exposed to two FEDs, one which delivered their preferred pellet (in the PR schedule), while the other delivered their non-preferred pellet at a lower cost (in a FR 5 schedule). Effortful pellet preference was conducted for one 24-h session. The number of nose-pokes for each pellet type and the percentage of animals that continued to work for their preferred pellet, even at an increasingly higher cost, were evaluated.
2.6. Spatial Learning and memory
Spatial learning and memory was evaluated using the Morris water maze as previously described (McKinney and Murphy, 2006), using Cohort 6 (n: B6 = 38 [16M, 22F]; S1 = 45 [23M, 22F]; Fig. 4). For one week prior to the start of the experiment, mice were handled once per day to habituate mice to the experimenter. The water maze consisted of a 1.2-m diameter pool filled with water maintained at 25 ± 2 °C, which was made opaque by non-toxic white paint. On the first day of the experiment, mice were given 6 visible platform trials, in which the platform was marked with a distinct local cue (flag on the platform); the platform location was moved after each set of 2 trials (3 unique locations total). Subsequently, for training (during which an unmarked platform was hidden approximately 1 cm below the surface of the water), mice were given 4 trials per day for 9 days, with the starting positions chosen pseudo-randomly each day. Trials ended when the mouse climbed onto the platform, with a maximum time of 60 s. To test memory for the platform location, after every 3 days of training (24 h after the last training trial in the set), a 60 s probe trial (with no platform present) was performed; a total of 3 probe trials were interspersed throughout training. After each probe trial, a pseudo-randomized, pre-determined subset of mice was removed from the experiment for terminal blood collection to analyze CORT concentration (for each group, n: B6 = 10 [5M, 5F]; S1 = 10 [5M, 5F]). All trials were recorded using a digital camera located above the pool connected to a desktop PC; Watermaze 4 software (Actimetrics, Evanston, IL) was used for data analysis.
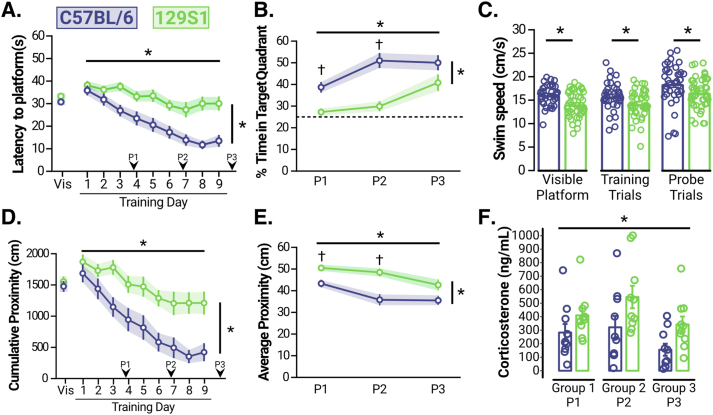
Fig. 4.
129S1 mice exhibit spatial learning and memory deficits and higher CORT levels in the Morris water maze.
(A) Prior to training, Visible Platform trials (Vis) revealed no differences between C57BL/6 (n = 38 [16M, 22F]) and 129S1 (n = 45 [23M, 22F]) mice (comprising Cohort 6) in the latency to swim to a marked platform (p = 0.1876). Analysis of training trials (Training Day 1–9) revealed that both C57BL/6 and 129S1 mice improved performance (decreased latency) with training (p < 0.0001), but that 129S1 mice were impaired relative to C57BL/6 mice (p < 0.0001). Arrows indicate the points at which a probe trial was given. (B) Analysis of probe trials revealed that memory for the platform location (percent time spent in the target quadrant, where the platform was previously located) improved over the course of training for both C57BL/6 and 129S1 mice (p < 0.0001), but that 129S1 mice exhibited memory deficits relative to C57BL/6 mice (p < 0.0001). Post-hoc analysis showed that 129S1 mice spent significantly less time in the target quadrant than C57BL/6 mice on Probe trials 1 and 2 (Probe 1, p = 0.0023; Probe 2, p < 0.0001) but exhibited equal performance by Probe 3 (p = 0.1035). The dashed line indicates “chance” performance level (25% time in the target quadrant). (C) 129S1 mice swam more slowly than C57BL/6 mice (average of all trials in each phase are shown; Visible Platform, p = 0.0004; Training Trials, p = 0.0123; Probe Trials, p = 0.0316). (D–E) Analysis using Gallagher's proximity measures (lower values reflect better performance) showed that, even when differences in swim speed are accounted for, both strains improved performance over the course of the experiment (Day of Training: D, p < 0.0001; Probe Number: E, p < 0.0001) but the 129S1 mice still exhibited impaired performance in both training trials (D, p = 0.0002) and probe trials (E, p < 0.0001). Further, post-hoc analysis showed that 129S1 mice exhibited significantly worse memory for the platform location compared to C57BL/6 mice on Probe trials 1 and 2 (Probe 1, p = 0.0084; Probe 2, p < 0.0001) but showed equal performance by Probe 3 (p = 0.0807). Arrows in D indicate the points at which a probe trial was given. (F) At the end of each probe trial, a pre-determined, randomized subset of animals (129S1: n = 10 [5 M, 5 F]; C57BL/6: n = 10 [5 M, 5 F] for Probe 1, Probe 2, and Probe 3) was removed from the experiment to measure CORT levels. Analysis showed that 129S1 mice exhibited higher CORT levels over the entire course of the water maze experiment compared to C57BL/6 mice (p < 0.0001). For all panels, asterisks indicate significant main effects and crosses indicate significant post-hoc tests.
2.7. Corticosterone assay
Stress levels were assessed by measuring plasma CORT levels. To evaluate the effects of acute stress in mice that had performed open-field exploration and novel tactile-object recognition (subset of Cohort 1), a period of elevated stress was induced by immobilizing mice in a restrainer (constructed of Plexiglass and Teflon cinched together by Velcro) for 30 min before samples were collected, as previously described (Stinnett et al., 2015). In animals that performed pellet preference and progressive ratio operant learning experiments (Cohort 3), blood samples were collected at the end of the experiment. Both of sample collections were carried out at 8 a.m., 3 h after the beginning of the light phase to coincide with the predicted low levels of circulating CORT based on diurnal neuroendocrine rhythms (Gong et al., 2015; Sollars et al., 2014). To assess CORT during water maze (Cohort 6), samples were collected directly following the indicated probe trial. In general, mice were euthanized by cervical dislocation followed by rapid decapitation to collect trunk blood, except during the operant learning experiments, in which blood was collected from live mice via a tail nick. In all cases, blood was deposited into pre-chilled Eppendorf tubes containing 2 μl of 0.5M EDTA to prevent coagulation. Tubes were centrifuged at 3000 rpm for 10 min at 4 °C, and plasma was collected and stored at −20 °C. CORT levels were assessed by colorimetric immunoassay using a CORT ELISA kit with a sensitivity of 27.0 pg/mL (Enzo Life Sciences, Farmingdale, NY), according to manufacturer directions. Because of the high sensitivity of this kit, we diluted our samples 1:60 so that they would fall within the linear range of the standard curve.
2.8. Statistical analyses
A balanced design was employed in each experiment using age-matched animals and approximately equal numbers of males and females; all animals were anonymized by tail-mark coding during experiments. Run order was pseudorandomized by strain and cage. Experimental parameters (such as the location of the FED on the back or front of the cage) were counterbalanced between strains for each experiment. Several animals were removed from their cohorts prior to analysis (and thus not reported in the cohort n elsewhere in the text or Supplemental Table 1): these included 2F S1 mice from Cohort 5, because of equipment malfunction, and 9 mice from Cohort 6 (B6 = 8 [7M, 1F]; S1 = 1F) because of nonperformance (failure to find the visible platform or floating during training and probe trials). Key statistical results are reported in the Results sections (in the form: test statistic[degrees of freedom], p value) for individual experiments; detailed statistical information is available in Supplementary Table 1. Unless otherwise noted, data are reported as mean ± standard error of the mean (SEM); graphs also show individual data points (mice) where applicable. Distribution normality was evaluated using the Shapiro-Wilk test, and parametric (F ANOVA, t tests) or non-parametric (Kruskal-Wallis, Kolmogorov-Smirnov, or Kaplan-Meier tests) statistical tests and post-hoc tests were applied as appropriate. While our experiments were not powered to evaluate sex as a statistical factor, all results have been stratified by sex and have been made available in an open-access data repository (figshare.com, https://doi.org/10.6084/m9.figshare.12462260.v1).
3. Results
3.1. 129S1 mice show reduced exploration in an open field and exhibit greater stress reactivity
Exploration of a novel environment is an intrinsically motivated behavior that is highly affected by stress in mice. For example, previous studies demonstrate that overall ambulatory behavior is strongly reduced by stress (Ihne et al., 2012; Marchette et al., 2018; Strekalova et al., 2004). Further, reduced time exploring the center of the open field (or thigmotaxis) has previously been used as an index for anxiety-like behavior in mice (LeBlanc et al., 2018; Simon et al., 1994). To establish the exploration tendencies of 129S1 and C57BL/6 mice, we compared their locomotor activity in an open-field arena (Fig. 1A) where they were allowed to freely explore for 10 min (for representative trajectories, see Fig. 1A1). Our results show that 129S1 mice traveled a shorter distance (Fig. 1A2; χ2 [1] = 27.054, p < 0.0001) and spent significantly less time in the center of the open field (Fig. 1A3; χ2[1] = 27.605, p < 0.0001) compared to C57BL/6 mice.
To determine if the exploration differences may be related to a strain-specific neuroendocrine stress response, we measured CORT levels in male mice after 30 min of restraint stress and in those that did not receive restraint stress (Fig. 1B). Our analyses revealed an interaction between strain and stress condition (F[1,58] = 16.94, p < 0.0001); the 129S1 mice exhibited higher CORT levels after the 30-min restraint stress than C57BL/6 mice (Fig. 1B, “Stressed”; t[58] = -0.033, p < 0.0001), but there was no difference in those that did not receive restraint stress (Fig. 1B, “Unstressed”; t[58] = -6.888, p = 0.9735), consistent with previous findings of a dysregulated HPA axis in 129S1 mice (Camp et al., 2012). To further investigate the link between exploration and a potentiated neuroendocrine stress response, we correlated CORT concentration with open field exploration measures (Fig. 1C and D). We found that mice with higher CORT levels following restraint stress traveled a shorter distance (Fig. 1C1; Pearson r = −0.572, t[39] = -4.36, p < 0.0001) and spent less time in the inner zone in the open field (Fig. 1C2; Pearson r = −0.380; t[39] = -2.5548, p = 0.0146). No significant correlations were observed in the unstressed condition between CORT levels and distance traveled (Fig. 1D1; Pearson r = −0.037, t[19] = -0.1625, p = 0.8726) or time spent in the inner zone (Fig. 1D2; Pearson r = −0.20, t[19] = -0.8902, p = 0.3845). These findings support the conclusion that a potentiated neuroendocrine stress response is associated with reduced exploration and increased anxiety-like behavior such as thigmotaxis in the 129S1 mice.
3.2. 129S1 mice exhibit reduced object and social exploration
In an effort to assess learning and memory in an appetitively motivated paradigm, mice were evaluated in a recognition memory test. This test takes advantage of a mouse's innate preference for novelty (Dere et al., 2007); memory for a previous experience is inferred when a mouse spends significantly more time exploring a new, unfamiliar entity compared to a familiar one. Given that 129S1 mice exhibited reduced exploration in the open field experiments, we selected entities that were predicted to highly engage mouse exploration and interaction, including inanimate objects with distinct textures (tactile-objects) (Watson et al., 2020; Wu et al., 2013) and social contacts (other mice) (Gould et al., 2014; Moy et al., 2004). However, similar to the results from the open field experiment, a majority of 129S1 mice exhibited drastically reduced exploration and spent very little (or no) time exploring tactile-objects or other mice (for exploration raster plots and representative trajectories, see Fig. 2A and B), which precluded our ability to evaluate memory in this paradigm.
Instead, we leveraged the data to evaluate differences between the strains in motivation to explore salient entities in their environment. We used the number of alternating visits to each tactile-object (Fig. 2C) or stimulus mouse (Fig. 2D) as a measure of exploration. In both cases, we found that there was a shift in the 129S1 population toward fewer alternations, indicating that they explore both tactile-objects (Fig. 2C1; D = 0.4370, p < 0.0001) and other mice (Fig. 2D1; D = 0.77778, p = 0.0119) significantly less than the C57BL/6 population. We further categorized exploration of tactile-objects (Fig. 2C2) and other mice (Fig. 2D2) based on the median number of alternations for the population (including all mice of both strains; 7 for tactile-objects, 23 for other mice) as low, moderate, or high explorers. We found that the majority of C57BL/6 mice were high explorers (76% for tactile-objects, 63% for other mice), while a plurality of the 129S1 mice were low explorers (68% for tactile-objects, 45% for other mice). While 129S1 mice have been shown to exhibit some subtle locomotor deficits that result in slower or less overall movement relative to C57BL/6 mice (Merritt and Rhodes, 2015; Serradj and Jamon, 2009), it is unlikely that these would solely account for the drastic difference in exploration (nearly half of 129S1 mice exhibit one or no alternations) over the entire 10-min trial. Further, similar to our results from the open field experiments, we found that higher CORT levels (measured after a 30-min restraint stress) were correlated with lower exploration of tactile-objects (Fig. 2E; Pearson r = -0.3016, t[37] = -1.9246, p = 0.0153). These results demonstrate that 129S1 mice not only exhibit reduced exploration of an open-field environment (see Fig. 1), but that they also demonstrate diminished motivation to explore novel tactile-objects or even investigate other mice, which have higher salience and intrinsic incentive value.
3.3. 129S1 mice exhibit reduced reward-seeking
While our experiments thus far show that the acute stress-susceptible 129S1 mice exhibit reduced exploration, the extent to which a reduction in reward-seeking contributes to the low exploration tendencies is unclear. To investigate this directly, we assessed differences in reward valuation and motivation between 129S1 and C57BL/6 mice (Fig. 3). Importantly, we conducted these experiments in a home-cage environment to reduce the novelty-induced suppression of exploration that we observed in the open field and tactile-object recognition experiments. Interestingly, while stress levels were lower overall in the home-cage environment (compared to Fig. 1B, “Unstressed), the 129S1 mice still exhibited higher CORT levels relative to the C57BL/6 (Fig. 3A; Cohort 3; F[1,22] = 12.34, p = 0.0020).
To determine whether 129S1 mice exhibit differential reward valuation, we measured their preference for standard chow vs. sweetened chocolate pellets (Fig. 3B; Cohort 3). Normally, mice prefer sweetened food or water, but when both types of pellets were available ad libitum (see Methods: Pellet Preference), 129S1 mice consumed significantly more chow pellets (Fig. 3B1, “Chow”; t[37.9] = 2.033, p = 0.049) and significantly fewer chocolate pellets (Fig. 3B1, “Choco”; t[37.9] = 4.038, p = 0.0003) than C57BL/6 mice. Further, a preference ratio (calculated for each mouse) showed that on an individual basis, 129S1 mice exhibited a much stronger preference for chow pellets than C56BL/6 mice (Fig. 3B2; F[1,22] = 15.48, p = 0.001), who consumed approximately equal amounts of each pellet type. These results suggest that 129S1 mice exhibit anhedonia, or reduced valuation of reward, since they display a greater preference for the chow over chocolate pellets relative to the C57BL/6 mice.
An alternative explanation for the strong chow pellet preference exhibited by the 129S1 mice is that they may be neophobic and, therefore, avoid the novel chocolate pellets. To address this, we repeated the pellet-preference experiment in a naïve cohort of mice (Cohort 4) and incorporated an extended (3 days) habituation period to both pellet types (Fig. 3C). After habituation, both strains exhibited greater preference for the chocolate pellets (Fig. 3B2 vs Fig. 3C2; main effect of Habituation: F[1,44] = 31.80, p < 0.0001), but 129S1 mice still consumed significantly more chow pellets (Fig. 3C1, “Chow”; t[42] = -2.019, p = 0.0499) and fewer chocolate pellets (Fig. 3C1, “Choco”; t[42] = 4.043, p = 0.0002) than C57BL/6 mice. Similarly, we find that the strain difference in preference ratio is maintained, indicating that individual 129S1 mice still exhibited a reduced preference for chocolate pellets compared to C57BL/6 mice (Fig. 3C2; F[1,22] = 10.42, p = 0.004). Altogether, these results support the conclusion that both strains exhibit some neophobia to the chocolate pellets (because their consumption can be increased by habituation to the pellets), but that the 129S1 mice also exhibit anhedonia (because regardless of habituation, they consume a lower proportion of the sweetened chocolate pellets than the C57BL/6 mice).
To directly assess differences in motivation, we measured the extent to which the original cohort of mice (Cohort 3) maintained nose-poking behavior for their preferred pellet type under a progressive ratio (PR) schedule of reinforcement (Fig. 3D). We found that 129S1 mice expended less effort (Fig. 3D1; F[1,21] = 14.36, p = 0.001) and exhibited a lower breakpoint (Fig. 3D2; F[1,21] = 7.17, p = 0.014) than C57BL/6 mice, suggesting that 129S1 mice are less motivated to work for their preferred pellet. It is important to note that this reduced responding in the 129S1 mice is unlikely to be due to differences in satiety, since both strains consume a comparable number of pellets when nose-pokes are not required for pellet delivery (129S1: 190.9 ± 18.7; C57BL/6: 167.5 ± 15.8; F[1,20] = 0.91, p = 0.352, data not graphed). Further, in a separate cohort of mice (Cohort 5), we assessed the effort each strain was willing to expend for their preferred pellet (on a PR schedule) when their non-preferred pellet was also available at a lower cost (FR5) (Fig. 3E). Our results show that while both strains expended equal effort for the lower-cost, non-preferred pellet (Fig E1, “NP”; t[34.4] = 0.515, p = 0.6095), the 129S1 mice expended significantly less effort for the preferred pellet (Fig E1, “PP”; t[34.4] = 3.114, p = 0.0037). In addition, when this choice differential was analyzed for individual mice, we found that the majority (67%) of 129S1 mice expended more effort for their non-preferred pellet, while only a small proportion (17%) of C57BL/6 mice did the same (Fig. 3E2). Taken together, these data show that 129S1 mice exhibit reduced reward-seeking characteristics, including neophobia, anhedonia, and amotivation, consistent with a stress-susceptible population.
3.4. 129S1 mice exhibit spatial learning and memory deficits in the Morris water maze
Our experiments thus far have not been able to distinguish between whether the stress-susceptible 129S1 mice merely exhibit reduced behavioral responsiveness and motivation to perform tasks, or whether their cognitive abilities are also negatively impacted. To directly assess differences in spatial learning and memory between 129S1 and C57BL/6 mice, we employed the Morris water maze (Fig. 4). This task is innately aversive to mice, who are ethologically non-aquatic, and imposes a direct negative experience, which is more likely to prompt action and allow evaluation of performance (thus avoiding the pitfalls of the tactile-object and social recognition memory paradigms).
Prior to training, we assessed potential disparities in non-cognitive abilities using a visible platform; no significant differences in latency to reach the visible platform were observed, suggesting that sensorimotor function, procedural learning, and motivation (in this task) were comparable between the strains (Fig. 4A, “Vis”; t[81] = 1.329, p = 0.1876). Next, animals were trained to find a hidden platform using spatial cues in the testing room for a total of 9 days (4 trials per day), with the starting positions chosen pseudo-randomly each day (Fig. 4A, “Training Day”). Our data indicate that both strains acquired the task because the latency to reach the platform decreased as training progressed (Fig 4A; F[5.669, 335.9] = 16.45, p < 0.0001). However, we also found a significant difference in performance between the strains (Fig 4A; F[1,81] = 21.37, p < 0.0001), suggesting that spatial learning is impaired in 129S1 mice relative to C57BL/6 mice. After every 3 days of training, a probe trial was given during which the platform was removed from the pool (for a total of 3 probe trials over the course of training); time spent in the quadrant where the platform was previously located (target quadrant) was quantified as a measure of memory for the platform location (Fig. 4A and B). Results from the probe trials (Fig. 4B) demonstrated that although memory for the platform location improved over the course of training for both strains (Fig 4B; F[2104] = 15.28, p < 0.0001), 129S1 mice exhibited memory deficits compared to C57BL/6 mice (Fig 4B; F[1,81] = 25.18, p < 0.0001). Further analysis revealed that 129S1 mice spent significantly less time in the target quadrant compared to C57BL/6 mice on Probe 1 (Fig 4B; t = 3.427[185], p = 0.0023) and Probe 2 (Fig 4B; t = 5.390[185], p < 0.0001) but displayed equal performance on Probe 3 (Fig 4B; t[185] = 2.130, p = 0.1035), suggesting 129S1 mice are impaired relative to the C57BL/6 mice (requiring more training to exhibit memory), but do eventually form a memory for the platform location. It is important to note that we found a significant difference in swim speed between strains in all phases of the water maze (Fig. 4C; Visible Platform, t[81] = 3.718, p = 0.0004; Training, t[81] = 2.562, p = 0.0123; and Probe t[81] = 2.188, p = 0.0316) which can confound the interpretation of results based purely on latency. Therefore, we also analyzed our data using Gallagher's proximity measures (Gallagher et al., 2015), which accounts for differences in swim speed, and confirmed that 129S1 mice were impaired in both learning during training trials (Fig 4D; F[1,81] = 14.70, p = 0.0002) and memory during probe trials (Fig 4E; F[1,81] = 17.34, p < 0.0001). This analysis also showed that 129S1 mice exhibited a memory impairment on Probe 1 (Fig 4E; t[185] = 3.028, p = 0.0084) and Probe 2 (Fig 4E; t[185] = 4.433, p < 0.0001), but were able to successfully form a memory for the platform location by Probe 3 (Fig 4E; t[185] = 2.231, p = 0.0807).
Immediately following each probe trial, a pre-determined, randomized subset of mice (Fig. 4F) was removed from the experiment to analyze CORT levels. Our results showed that 129S1 mice had significantly higher CORT levels compared to C57BL/6 over the course of the water maze experiment (F[1,73] = 13.04, p < 0.001), although there was no interaction between strain and probe trial number (F[3,73] = 0.97, p = 0.414). Thus, a potentiated neuroendocrine stress response is associated with spatial learning and memory deficits in the stress-susceptible 129S1 mice.
4. Discussion
Neuropsychiatric disorders like post-traumatic stress disorder (PTSD) are associated with heightened or prolonged physiological reactions to real or perceived stressors. These physiological reactions can affect multiple cognitive and behavioral domains, resulting in persistent negative emotions, diminished interest in previously rewarding activities, social detachment, and impairments in learning and memory (Baldi and Bucherelli, 2005; Sandi and Haller, 2015; Stanton et al., 2019; Yehuda and LeDoux, 2007). To elucidate the underlying mechanisms and identify effective therapeutic interventions, animal models that encompass many or all of these wide-ranging effects are needed. Previous studies have shown that 129S1 mice are inherently more susceptible to acute stressors compared to other strains, and that they exhibit persistent fear which is resistant to extinction in associative fear paradigms (Camp et al, 2009, 2012; Hefner et al., 2008). However, they have not been fully evaluated in other RDoCs (Insel et al., 2010), such as positive valence, sociability, arousal, and/or cognition, which align with alterations observed in anxiety-related disorders (Fenster et al., 2018). Our current work addresses this knowledge gap by evaluating 129S1 mice in distinct paradigms to designed to assess these disparate criteria. We find that 129S1 mice exhibit deficits across many RdoCs, including reduced exploration of environments, objects, and other mice, reduced reward-seeking and motivation to work for reward, and deficits in spatial learning and memory, and that these deficits are correlated with a potentiated neuroendocrine response.
Interestingly, many of the phenotypes we observed in 129S1 mice reflect behaviors that are known to be sensitive to disruption by stressful experiences or by exogenously elevating stress hormones in rodents. For example, stress resulting from repeated social defeat reduced distance traveled in an open field (Kudryavtseva et al., 1991) while chronic restraint stress or exogenous elevation of glucocortocoids resulted in novelty-aversion and impaired sociability (Vargas-López et al., 2015; Zain et al., 2019). The normal preference for sweetened food or water is highly susceptible to stress (Pothion et al., 2004; Strekalova et al., 2004) and reduced sucrose preference has often been used to identify stress-susceptible populations (Cao et al., 2010; Friedman et al., 2014). Likewise, social stress, restraint stress, and increased CORT levels reduced reward-seeking, lowered motivation to work for reward, and biased decision making towards a low reward/low effort choice (relative to a high reward/high effort choice) (Bryce and Floresco, 2016; Kúkel'ová et al., 2018; Wanat et al., 2013). Finally, both social stress and elevation of CORT affected different forms of learning and memory, including working memory and spatial memory (Bodnoff et al., 1995; Dominguez et al., 2019).
While it is possible that the deficits in different domains observed in the 129S1 mice are mediated by independent and distinct mechanisms, a more parsimonious model posits that the heightened stress response may represent a common underlying mechanism that is the ultimate driver of these behavioral impairments. For example, stress has been shown to affect brain regions involved in both reward-related behavior and learning and memory (Cabib and Puglisi-Allegra, 2012; Lowery-Gionta et al., 2018). In the nucleus accumbens chronic or prolonged stress blunts dopaminergic neurotransmission (Rossetti et al., 1993; Ventura et al., 2002) and leads to behavioral stress-coping failures, such as learned helplessness and decreased operant responding (Azzinnari et al., 2014; Bergamini et al., 2016). In the hippocampus, a stress-mediated elevation in glucocorticoids leads to alterations in both structure and function, including remodeling of dendritic arbors (Woolley et al., 1990), changes in neuronal plasticity (like attenuation of long-term potentiation (Pavlides et al., 1995)), and deficits in hippocampal-dependent learning and memory (Bodnoff et al., 1995; Dominguez et al., 2019). Taken together, the innately heightened stress susceptibility in 129S1 mice may mimic the effects of chronic stress exposure in other mouse strains, resulting in a reduction of both intrinsic (exploration of environments, objects, and other mice) and extrinsic (working for sweetened chocolate pellets) motivation-driven behaviors, as well as impairments in hippocampus-dependent spatial learning and memory.
Future studies will be needed to directly assess the role of a potentiated stress response in the myriad behavioral deficits observed in the 129S1 mice. Of particular interest will be whether modulating stress levels (either behaviorally or pharmacologically) is sufficient to rescue deficits in disparate domains concomitantly, or whether these behaviors rely on distinct and non-overlapping mechanisms. In addition, it will be important to determine to what extent a deficit in one domain affects performance in another domain. For example, a reduction in motivation may affect performance in a learning and memory task (as was the case in our novel tactile-object and social recognition paradigms). These complexities necessitate the use of multiple paradigms and careful experimental design to reduce interpretation confounds and examine behavior in an integrative context. Overall, our study shows that a diversity of behavioral alterations exists in stress-susceptible 129S1, including reduced exploration, amotivation, anhedonia, neophobia, and cognitive impairments. Thus, the 129S1 mice can serve as a model to enable the mapping of distinct and overlapping neural mechanisms that tie dysregulated stress reactivity to novelty-motivated exploration, reward-seeking, and cognitive performance. Further, this model may elucidate novel therapeutic treatments such as behavioral interventions, pharmacological agents, and epigenetic modulators to ameliorate human psychopathology.
Funding
National Institutes of General Medical Sciences (GM111725, GM086262); National Institutes on Aging (AG052934, AG024824); Strategic Translational Research Award, Depression Center, Michigan Medicine, University of Michigan, Ann Arbor MI.
CRediT authorship contribution statement
G. Rodriguez: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. S.J. Moore: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Project administration, Supervision, Funding acquisition. R.C. Neff: Methodology, Investigation, Writing - original draft, Writing - review & editing. E.D. Glass: Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. T.K. Stevenson: Investigation, Formal analysis, Writing - review & editing. G.S. Stinnett: Methodology, Investigation, Writing - review & editing. A.F. Seasholtz: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. G.G. Murphy: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. V.A. Cazares: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Supervision, Funding acquisition.
Declaration of competing interest
N/A.
Acknowledgments
The authors thank Dr. Marieke Gilmartin for helpful discussions regarding this project. We also thank Dr. Alexxai Kravitz and Dr. Amy Sutton for help implementing and troubleshooting the Feeding Experimental Devices (FED) used for operant learning experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100262.
Contributor Information
G.G. Murphy, Email: murphyg@umich.edu.
V.A. Cazares, Email: vac1@williams.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Appel K., Schwahn C., Mahler J., Schulz A., Spitzer C., Fenske K., Stender J., Barnow S., John U., Teumer A., Biffar R., Nauck M., Völzke H., Freyberger H.J., Grabe H.J. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzinnari D., Sigrist H., Staehli S., Palme R., Hildebrandt T., Leparc G., Hengerer B., Seifritz E., Pryce C.R. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology. 2014;85:328–341. doi: 10.1016/j.neuropharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- Baldi E., Bucherelli C. The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity Biol. Toxicol. Med. 2005;3:9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G., Sigrist H., Ferger B., Singewald N., Seifritz E., Pryce C.R. Depletion of nucleus accumbens dopamine leads to impaired reward and aversion processing in mice: relevance to motivation pathologies. Neuropharmacology. 2016;109:306–319. doi: 10.1016/j.neuropharm.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff S., Humphreys A., Lehman J., Diamond D., Rose G., Meaney M. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 1995;15:61–69. doi: 10.1523/jneurosci.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C., Floresco S. Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41:2147–2159. doi: 10.1038/npp.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Camp M., Macpherson K., Lederle L., Graybeal C., Gaburro S., Debrouse L., Ihne J., Bravo J., O'Connor R., Ciocchi S., Wellman C., Lüthi A., Cryan J., Singewald N., Holmes A. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012;37:1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp M., Norcross M., Whittle N., Feyder M., D'Hanis W., Yilmazer‐Hanke D., Singewald N., Holmes A. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Gene Brain Behav. 2009;8:744–752. doi: 10.1111/j.1601-183x.2009.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Covington H., Friedman A., Wilkinson M., Walsh J., Cooper D., Nestler E., Han M. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:16453–16458. doi: 10.1523/jneurosci.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazares V.A., Rodriguez G., Parent R., Ouillette L., Glanowska K.M., Moore S.J., Murphy G.G. Environmental variables that ameliorate extinction learning deficits in the 129S1/SvlmJ mouse strain. Gene Brain Behav. 2019;19 doi: 10.1111/gbb.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W.E., Herman J.P., Watson S.J. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Daviu N., Bruchas M., Moghaddam B., Sandi C., Beyeler A. Neurobiological links between stress and anxiety. Neurobiol. Stress. 2019;11:100191. doi: 10.1016/j.ynstr.2019.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E., Huston J., Silva M. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dominguez G., Henkous N., Prevot T., David V., Guillou J., Belzung C., Mons N., Béracochéa D. Sustained corticosterone rise in the prefrontal cortex is a key factor for chronic stress-induced working memory deficits in mice. Neurobiol. Stress. 2019;10:100161. doi: 10.1016/j.ynstr.2019.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster R., Lebois L., Ressler K., Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci. 2018;19:535–551. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P., Whittle N., Flynn S., Graybeal C., Pinard C., Gunduz-Cinar O., Kravitz A., Singewald N., Holmes A. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol. Learn. Mem. 2014;113:69–81. doi: 10.1016/j.nlm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Walsh J., Juarez B., Ku S., Chaudhury D., Wang J., Li X., Dietz D., Pan N., Vialou V., Neve R., Yue Z., Han M. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Sci New York N Y. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Burwell R., Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 2015;129:540–548. doi: 10.1037/bne0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Miao Y., Jiao G., Sun M., Li H., Lin J., Luo M., Tan J. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PloS One. 2015;10 doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G., Burke T., Osorio M., Smolik C., Zhang W., Onaivi E., Gu T., DeSilva M., Hensler J. Enhanced novelty-induced corticosterone spike and upregulated serotonin 5-HT1A and cannabinoid CB1 receptors in adolescent BTBR mice. Psychoneuroendocrinology. 2014;39:158–169. doi: 10.1016/j.psyneuen.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., Brockway E., Lederle L., Wilcox T., Halladay L.R., Ding Y., Oh H., Busch E.F., Kaugars K., Flynn S., Limoges A., Bukalo O., MacPherson K.P., Masneuf S., Pinard C., Sibille E., Chesler E.J., Holmes A. Identification of a novel gene regulating amygdala-mediated fear extinction. Mol. Psychiatr. 2018;36:1. doi: 10.1038/s41380-017-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailwood J., Heath C., Robbins T., Saksida L., Bussey T. Validation and optimisation of a touchscreen progressive ratio test of motivation in male rats. Psychopharmacology. 2018;235:2739–2753. doi: 10.1007/s00213-018-4969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K., Whittle N., Juhasz J., Norcross M., Karlsson R., Saksida L., Bussey T., Singewald N., Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:8074–8085. doi: 10.1523/jneurosci.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E., Morano M.I., Akil H., Watson S.J. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical Axis. J. Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Holmes A., Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihne J., Fitzgerald P., Hefner K., Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology. 2012;62:464–473. doi: 10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol. Psychiatr. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Han M., Graham D., Berton O., Renthal W., Russo S., Laplant Q., Graham A., Lutter M., Lagace D., Ghose S., Reister R., Tannous P., Green T., Neve R., Chakravarty S., Kumar A., Eisch A., Self D., Lee F., Tamminga C., Cooper D., Gershenfeld H., Nestler E. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krueger J., Moore S., Parent R., McKinney B., Lee A., Murphy G. A novel mouse model of the aged brain: over-expression of the L-type voltage-gated calcium channel CaV1.3. Behav. Brain Res. 2016;322:241–249. doi: 10.1016/j.bbr.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva N., Bakshtanovskaya I., Koryakina L. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kúkel’ová D., Bergamini G., Sigrist H., Seifritz E., Hengerer B., Pryce C. Chronic social stress leads to reduced gustatory reward salience and effort valuation in mice. Front. Behav. Neurosci. 2018;12:134. doi: 10.3389/fnbeh.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc K., London T., Szczot I., Bocarsly M., Friend D., Nguyen K., Mengesha M., Rubinstein M., Alvarez V., Kravitz A. Striatopallidal neurons control avoidance behavior in exploratory tasks. Mol. Psychiatr. 2018;25:491–505. doi: 10.1038/s41380-018-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M., Neufeld-Cohen A., Kuperman Y., Tsoory M., Gil S., Chen A. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:6906–6916. doi: 10.1523/jneurosci.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H., Roseberry K., Levey D.F., Rogers J., Kosary K., Prabha S., Jones T., Judd S., McCormick M.A., Wessel A.R., Williams A., Phalen P.L., Mamdani F., Sequeira A., Kurian S.M., Niculescu A.B. Towards precision medicine for stress disorders: diagnostic biomarkers and targeted drugs. Mol. Psychiatr. 2019;25:918–938. doi: 10.1038/s41380-019-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S., Flagel S. A proposed role for glucocorticoids in mediating dopamine-dependent cue-reward learning. Stress. 2020:1–41. doi: 10.1080/10253890.2020.1768240. Amsterdam Neth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta E.G., Crowley N.A., Bukalo O., Silverstein S., Holmes A., Kash T.L. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology. 2018;139:68–75. doi: 10.1016/j.neuropharm.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette R., Bicca M., Santos E.C., Lima Tcm. Distinctive stress sensitivity and anxiety-like behavior in female mice: strain differences matter. Neurobiol. Stress. 2018;9:55–63. doi: 10.1016/j.ynstr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A., Mamidanna P., Cury K., Abe T., Murthy V., Mathis M., Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018;21:1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- McEwen B., Akil H. 2020. Revisiting the Stress Concept: Implications for Affective Disorders.https://www.jneurosci.org/content/40/1/12.abstract?etoc [WWW Document]. URL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B., Gianaros P. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney B., Murphy G. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem Cold Spring Harb N Y. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S., Ressler K., Koenen K., Neylan T., Germine L., Jovanovic T., Clifford G., Zeng D., An X., Linnstaedt S., Beaudoin F., House S., Bollen K., Musey P., Hendry P., Jones C., Lewandowski C., Swor R., Datner E., Mohiuddin K., Stevens J., Storrow A., Kurz M., McGrath M., Fermann G., Hudak L., Gentile N., Chang A., Peak D., Pascual J., Seamon M., Sergot P., Peacock W., Diercks D., Sanchez L., Rathlev N., Domeier R., Haran J., Pearson C., Murty V., Insel T., Dagum P., Onnela J., Bruce S., Gaynes B., Joormann J., Miller M., Pietrzak R., Buysse D., Pizzagalli D., Rauch S., Harte S., Young L., Barch D., Lebois L., Rooij S.J.H., Luna B., Smoller J., Dougherty R., Pace T., Binder E., Sheridan J., Elliott J., Basu A., Fromer M., Parlikar T., Zaslavsky A., Kessler R. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatr. 2019;25:283–296. doi: 10.1038/s41380-019-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J.R., Rhodes J.S. Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze. Behav. Brain Res. 2015;280:62–71. doi: 10.1016/j.bbr.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Deshpande K., Stinnett G., Seasholtz A., Murphy G. Conversion of short-term to long-term memory in the novel object recognition paradigm. Neurobiol. Learn. Mem. 2013;105:174–185. doi: 10.1016/j.nlm.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.J., Murphy G.G., Cazares V.A. Turning strains into strengths for understanding psychiatric disorders. Mol. Psychiatr. 2020:1–14. doi: 10.1038/s41380-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy S., Nadler J., Barbaro R., Johns J., Magnuson T., Crawley J. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Gene Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nguyen K., O'Neal T., Bolonduro O., White E., Kravitz A. Feeding Experimentation Device (FED): a flexible open-source device for measuring feeding behavior. J. Neurosci. Methods. 2016;267:108–114. doi: 10.1016/j.jneumeth.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez J.R., Anderton C.R., Renslow R.S. Optimizing colormaps with consideration for color vision deficiency to enable accurate interpretation of scientific data. PloS One. 2018;13 doi: 10.1371/journal.pone.0199239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C., Watanabe Y., Magariños A.M., McEwen B.S. Opposing roles of type I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S., Bizot J., Trovero F., Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Quervain D., Schwabe L., Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 2016;18:7–19. doi: 10.1038/nrn.2016.155. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Carboni L., Andreoli M., Ballottari A., Arban R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav. Brain Res. 2010;216:100–108. doi: 10.1016/j.bbr.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Richardson N., Roberts D. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 2004;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rossetti Z.L., Lai M., Hmaidan Y., Gessa G.L. Depletion of mesolimbic dopamine during behavioral despair: partial reversal by chronic imipramine. Eur. J. Pharmacol. 1993;242:313–315. doi: 10.1016/0014-2999(93)90257-i. [DOI] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sanislow C., Ferrante M., Pacheco J., Rudorfer M., Morris S. Advancing translational research using NIMH research domain criteria and computational methods. Neuron. 2019;101:779–782. doi: 10.1016/j.neuron.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Serradj N., Jamon M. The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behav. Brain Res. 2009;201:59–65. doi: 10.1016/j.bbr.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Simon P., Dupuis R., Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Sollars P., Weiser M., Kudwa A., Bramley J., Ogilvie M., Spencer R., Handa R., Pickard G. Altered entrainment to the day/night cycle attenuates the daily rise in circulating corticosterone in the mouse. PloS One. 2014;9 doi: 10.1371/journal.pone.0111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R., Deak T. A users guide to HPA axis research. Physiol. Behav. 2017;178:43–65. doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C., Holmes A., Chang S., Joormann J. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42:23–42. doi: 10.1016/j.tins.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett G., Westphal N., Seasholtz A. Pituitary CRH-binding protein and stress in female mice. Physiol. Behav. 2015;150:16–23. doi: 10.1016/j.physbeh.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T., Spanagel R., Bartsch D., Henn F., Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004;29 doi: 10.1038/sj.npp.1300532. 2007–17. [DOI] [PubMed] [Google Scholar]
- Temme S., Bell R., Pahumi R., Murphy G. Comparison of inbred mouse substrains reveals segregation of maladaptive fear phenotypes. Front. Behav. Neurosci. 2014;8:282. doi: 10.3389/fnbeh.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-López V., Torres-Berrio A., González-Martínez L., Múnera A., Lamprea M. Acute restraint stress and corticosterone transiently disrupts novelty preference in an object recognition task. Behav. Brain Res. 2015;291:60–66. doi: 10.1016/j.bbr.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Vasterling J., Duke L., Brailey K., Constans J., Allain A., Sutker P. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Ventura R., Cabib S., Puglisi-Allegra S. Genetic susceptibility of mesocortical dopamine to stress determines liability to inhibition of mesoaccumbens dopamine and to behavioral ‘despair’ in a mouse model of depression. Neuroscience. 2002;115:999–1007. doi: 10.1016/s0306-4522(02)00581-x. [DOI] [PubMed] [Google Scholar]
- Wanat M., Bonci A., Phillips P. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L., Stone T., Williams D., Williams A., Sims-Robinson C. High-fat diet impairs tactile discrimination memory in the mouse. Behav. Brain Res. 2020;382:112454. doi: 10.1016/j.bbr.2019.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T., Miller E., Bache S., Müller K., Ooms J., Robinson D., Seidel D., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K., Yutani H. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- Woolley C., Gould E., McEwen B. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wu H., Ioffe J., Iverson M., Boon J., Dyck R. Novel, whisker-dependent texture discrimination task for mice. Behav. Brain Res. 2013;237:238–242. doi: 10.1016/j.bbr.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Yehuda R., LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zain M., Pandy V., Majeed A., Wong W., Mohamed Z. Chronic restraint stress impairs sociability but not social recognition and spatial memory in C57BL/6J mice. Exp Anim Tokyo. 2019;68:113–124. doi: 10.1538/expanim.18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Kohen R., Parent R., Duan Y., Fisher G., Korn M., Ji L., Wan G., Jin J., Püschel A., Dolan D., Parent J., Corfas G., Murphy G., Giger R. PlexinA2 forward signaling through Rap1 GTPases regulates dentate gyrus development and schizophrenia-like behaviors. Cell Rep. 2018;22:456–470. doi: 10.1016/j.celrep.2017.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.