Abstract
Background:
Telerehabilitation (TR) in chronic stroke patients has emerged as a promising modality to deliver rehabilitative treatment-at-home. The primary objective of our methodical clinical study was to determine the efficacy of a novel rehabilitative device in terms of recovery of function in daily activities and patient satisfaction and acceptance of the medical device provided.
Methods:
A 12-week physiotherapy program (balance exercises, upper and lower limb exercises with specific motor tasks using a biofeedback system and exergaming) was administered using the WeReha device. Twenty-five (N = 25) chronic stroke outpatients were enrolled, and the data of 22 patients was analyzed. Clinical data and functional parameters were collected by Berg Balance scale (BBS), Barthel Index (BI), Fugl-Meyer scale (FM), Modified Rankin scale (mRS), and Technology Acceptance Model (TAM) questionnaire at baseline (T0), after treatment (T1), and at the 12-week follow-up (T2). Statistical tests were used to detect significant differences (P < .05), and Cohen’s (Co) value was calculated.
Results:
BI scores improved significantly after treatment (P = .036; Co 0.776, medium), as well as BBS scores (P = .008; Co 1.260, high). The results in FM scale (P = .003) and mRS scores (P = .047) were significant post treatment. Follow-up scores remained stable across all scales, except the BI. The A and C sub-scales of the TAM correlated significantly to only a T2 to T1 difference for BI scores with P = .021 and P = .042.
Conclusion:
Currently, the WeReha program is not the conventional therapy for stroke patients, but it could be an integrative telerehabilitative resource for such patients as a conventional exercise program-at-home.
ClinicalTrials.gov identifier: NCT03964662.
Keywords: Neurology, physical therapy, rehabilitation, stroke, telerehabilitation, virtual reality
Introduction
Strokes are a major cause for disabilities worldwide. Each year, an estimated 15 million people globally suffer a stroke, of whom 5 million die and another 5 million remain affected with serious disabilities. Approximately 20% of stroke survivors are aged under 65 years and thus, in most cases, are active in their professional careers.1,2
After a stroke, restricted participation in social and work activities (mainly in younger patients aged <65 with social and economic repercussions) was observed.3
Additionally, stroke-related sequelae range from associated motor deficit, often to cognitive impairment, which contributes to a reduction in daily activities with a low level of participation and a poor quality of life. Rehabilitation strategies focus on the recovery of autonomy in both simple and complex daily activities. The reduction of disability should be developed so as to improve the quality of life.3,4 Moreover, rehabilitation is one of the most important aspects of patient care after a stroke and has proven to be fundamental to the degree of recovery and level of independence after hospitalization.4 Consequently, the provision of comprehensive rehabilitation programs with adequate duration and resources is essential in stroke care rehabilitation and should be prioritized in the acute and chronic phases.5,6 Furthermore, good communication and shared decision-making with patients and their family (or caregiver) is critical for high-quality post-stroke rehabilitative care: in order to be more satisfied with both clinical and rehabilitative treatment proposals the patient must be personally involved in all decisions made, feeling and being the protagonist of every therapeutic choice.7
Patients with mild or moderate disability and who are medically stable can continue rehabilitation at home with supported discharge teams early instead of a prolonged hospital stay.8,9 Advanced novel rehabilitation therapies provide (or yield) multi-sensory stimulation, and dual-task exercises, in addition to standard rehabilitation, are recommended for stroke patients, 6 months after the stroke. For example, visual feedback in a virtual environment-based treatment, with conventional rehabilitation, compared to conventional rehabilitation alone, produces better outcomes for upper limbs, regardless of the etiology of the stroke.10 Similarly, exergaming for post-stroke upper extremity rehabilitation demonstrated a good result in a home-based telerehabilitation setting.11–13
Psychological problems are common following a stroke, causing stroke survivors to lack the motivation to take part in exercise training. Thus, rehabilitation courses that promote motivation and satisfaction of the patient and caregiver must be included to improve activities of daily living. Patient compliance with the rehabilitation program is fundamental for a successful outcome.14 Furthermore, the use of technology should make the rehabilitation experience pleasant, fun, and satisfying compared to conventional therapy by motivating patients to adhere to the rehabilitative treatment.15,16 Gaming exercises increase the efficacy of telerehabilitation (TR) programs through greater patient motivation, better learning through repetition in an enriched environment (such as virtual reality), confidence through reinforcement and ease of feedback, and positivity through achievement and social interaction.15 TR is an emerging way to deliver rehabilitative treatment remotely and is a new rehabilitative approach, especially in recent years, which allows the patient and caregiver to interact easily and rather quickly via internet connection with the doctor or other professionals.16 The positive approach of patients towards technology also stimulates more daily motor activity beyond the treatment sessions, improving their quality of life. The function of stroke patients after the stroke period treatment improves with technological devices, particularly for upper limb and hand recovery.17–19
During rehabilitation after a stroke, patients require the input of several skilled health care personnel, including rehabilitation physicians, physiotherapists, speech therapists, and occupational therapists but these resources are often unavailable, impeding the recovery from physical limitations among stroke survivors. Thus, technology in home-base rehabilitation is generally considered a positive factor for hospitals and the community, economically and logistically, and, when integrated with conventional rehabilitation protocols, increases activities that are vital for reducing stroke-related disabilities,20 given that after a stroke, even if mild, patients experience reduced physical activity.
Nevertheless, recovery of the upper extremities has often been reported several years after the stroke, an improvement that is likely mediated by a complex combination of spontaneous and learning-dependent processes, including restitution, substitution, and compensation.21,22 In particular, upper extremity impairments have chronic effects on functional independence and satisfaction in 50-70% of all stroke patients.21,22 Also, home-based TR with distance support is a viable approach to meet the rehabilitative needs of stroke survivors, having comparable or greater effects on motor, higher cortical, and mood disorders compared with conventional face-to-face therapy.23–25
As previously stated, the hypothesis of our research was that a home-based rehabilitation, remotely supervised by a rehabilitation physician and physiotherapist, with innovative technology systems named WeReha, has the patient using wearable sensors and biofeedback to monitor total body exercises and “smart objects” for hand rehabilitation. For patients who have suffered from a chronic stroke, it may be a good resource for the recovery of disabilities, as long as the patient openly accepts the proposed technology.
Considering this premise, our clinical study aims to determine the efficacy of this novel rehabilitative device “WeReha”, for in-home rehabilitation in chronic stroke patients with regards to recovery of function and disability as the focused outcome followed by satisfaction and acceptance of this medical device by patients as the secondary outcome. Furthermore, to this end, we’ve posed the following question: “Could the patient’s acceptance of this medical device influence the outcome of their rehabilitation?”
Materials and Methods
Participants
Patients were recruited from the outpatient neurological rehabilitation service of IRCSS Bonino Pulejo (Italy) and the rehabilitation service of G. D’Annunzio University of Chieti (Italy).
Inclusive criteria was: age between 18 and 85 years; having experienced their first episode of an ischemic stroke, as documented by computed tomography (CT) or magnetic resonance imaging (MRI); the ability to remain seated with their feet on the ground for at least 45 min in a comfortable chair containing a backrest and armrests; modified Rankin scale (MRS)26 ⩽2 with the ability to walk independently indoors without any aid or with aid and caregiver supervision; Mini Mental State Examination (MMSE)27 ⩾24; ability and willingness to participate in the study; the absence of a serious comorbidity with respect to other balance disorders. All patients had been stabilized with regard to pharmacological therapy for at least 3 months before being enrolled. All patients gave and signed their consent to participate in the study.
Patients were excluded if they reported previous strokes; the presence of serious pathologies, such as heart or oncological disease; a predisposition to psychological or neurological disorders; bilateral weakness of the upper limbs and a Modified Ashworth Scale (MAS) > 2; aphasia with consequent serious difficulties in communication; cognitive deficits; shoulder-hand syndrome following stroke; orthopedic pathologies or other neurological conditions that influence cognitive abilities and motor skills (e.g., fractures and extrapyramidal symptoms); and participation in another clinical study or rehabilitation therapy. No additional traditional treatments were administered during the WeReha rehabilitation.
The presence of a caregiver was strongly recommended, but if the patient could not provide one, for ethical reasons and to ensure rehabilitative care, the patient was not excluded from the treatment.
This study was performed per the Helsinki Declaration on human experimentation and was approved by the local ethic committee of G. D’Annunzio University of Chieti—Italy (protocol number 1827). Furthermore, this study is a part of the Magic Project registered at the ClinicalTrial.gov Register [NCT03964662]. All patients signed informed consent forms after receiving detailed information about the study’s aim and procedures. The rights of human subjects who were involved in the study were protected.
Study design and data collection
We used the single-subject design model, which is suitable for research in rehabilitation per the TRENDS guidelines.28,29 Three phases of the protocol were defined as: pre-treatment, post-treatment, and follow-up.
Before starting the treatment, all patients underwent a neurological and rehabilitation examination. At the end of the visit, the rating scales were administered by the examining physicians.
The assessments were performed at baseline (T0, within 1 week before the treatment outset), post-training sessions (T1, 3 months), and 12 weeks after rehabilitation (T2, follow-up).
Each patient was monitored for a total of 6 months. During the rehabilitative training, existing pharmacological regimens remained constant, and the 3 assessments were performed under similar medical conditions.
Outcome Measures
Berg balance scale (BBS)
The BBS is a 14-item scale that measures static and dynamic balance.30 It contains items that require the respondents to maintain positions of varying difficulty and perform tasks, such as standing and sitting unsupported, making transfers, turning to look over the shoulders, and alternating their feet on a stool. Each task is scored on a 4-point ordinal scale from 0 to 4, for a maximum score of 56 (range 0-56). Even patients who obtain a high score (53 or 54) only have moderate assurance that they are not at risk for a fall in the upcoming several months.
Balance capacity was defined as the ability to maintain, achieve, or restore a state of balance during any posture.31 This parameter includes all aspects of balance capacity, as described in a model by Tyson et al.,32 such as static and dynamic balance, body alignment, and weight distribution. The balance outcomes in this study assessed any of these aspects and have been validated and have been found to be reliable for individuals with stroke.
Barthel index (BI)
The BI comprises 10 items to evaluate autonomy respect to the activities of daily living (ADL): “feeding,” “bathing,” “grooming,” “dressing,” “bowel” and “bladder control,” “toilet use,” “transfers (bed to chair and back),” “mobility,” and “stair climbing.” The items are rated as whether patients can perform the activities independently or with assistance or are totally dependent (scored 10, 5, or 0 respectively, or from 15 to 0 for transfers and mobility).33,34
Fulg-Meyer scale (FM)
The FM scale is a 226-point multi-item Likert-type scale that was developed as an evaluative measure of recovery from hemiplegic stroke. It is divided into 5 domains: motor function, sensory function, balance, joint range of motion, and joint pain. Each domain contains multiple items, each of which is scored on a 3-point ordinal scale (0 = cannot perform, 1 = performs partially, 2 = performs fully). The total possible scale score is 226, and points are divided among the domains as follows: for the motor score, we have ranges from 0 (hemiplegia) to 100 points (normal motor performance), divided into 66 points for upper extremity and 34 points for the lower extremity; for the sensation, we have ranges from 0 to 24 points, divided into 8 points for light touch and 16 points for position sense; for balance, we have ranges from 0 to 14 points, divided into 6 points for sitting and 8 points for standing; for joint range of motion, we have ranges from 0 to 44 points; for joint pain, we have ranges from 0 to 44 points.35,36
Modified Rankin scale (mRS)
The modified Rankin Scale (mRS) is a clinician-reported measure of global disability that has been applied widely for evaluating recovery from stroke. It is now the most commonly used functional measure in stroke trials and has been the primary or co-primary outcome in most recent large-scale stroke trials. The scale runs from 0 to 6, running from perfect health without symptoms to death.26,37
Technology acceptance model (TAM) questionnaire
The TAM explains the determinants of computer acceptance, based on a Likert scale, where 1 denotes “I don’t agree” and 7 indicates “I completely agree”; for convenience, the 4 main items were assigned letters: A, perceived ease of use (scored from 7 to 49); B, perceived utility (6-42); C, attitude toward new technologies (5-35); and D, attitude toward the use of new technologies (4-28). The scores achieved in the different items are summed to have a total score, in which the maximum score, corresponding to 154, indicates a complete acceptance of the technology device for the rehabilitation process, and the minimum score, corresponding to 22, indicates a tendency to refuse technology. The TAM scale was administered once, at T1.
A key purpose of the TAM is thus to provide a basis for tracing the impact of external factors on internal beliefs, attitudes, and intentions. The TAM was formulated to achieve these goals by identifying a small number of fundamental variables that have been suggested by previous research on the cognitive and affective determinants of computer acceptance by patients as a rehabilitation tool.38,39
Rehabilitative intervention
The WeReha (CoRehab Srl-Italy) device comprises: (i) an Android tablet with dedicated software; (ii) an inertial Bluetooth sensor that is equipped with an accelerometer, gyroscope, and magnetometer; (iii) a kit of elastic bands with a velcro pocket that allows on to wear the sensor on specific segments of the body (chest, thigh, foot, and wrist); and (iv) an assortment of objects that have been printed on a 3-dimensional printer (an hourglass, a remote control, and a joystick) that become “animated” tools (Smart Object) through the use of indoor sensors.
The WeReha software presents the patient with a series of rehabilitation exercises in the form of interactive games, using the sensor that is applied to various body segments or the Smart Objects.
Based on its inertial sensor, the WeReha records every movement by the body segment on which the sensor is positioned or by the Smart Object into which the sensor is inserted.
The exercises that are implemented in WeReha were designed by rehabilitation physicians (n = 3) and physiotherapists (n = 3) who are experts in rehabilitation after stroke. These interactive exercises are for the lower and upper limbs as well as for the head and trunk particularly with respect to trunk balance.40,41 They follow simple and safe principles, which make them suitable for a home setting. Such exercises required the patients to be in a sitting or standing position, with or without support, with constant support (e.g. a table, wall or chair). If the patient did not have the possibility to be supervised by the caregiver, for safety, the device could adapt to the difficulty of the exercise being performed. In fact, every time the patient started a new session the device would ask them “how do you feel today?” and “is there anyone with you during the session today?” to calibrate exercises correctly.
Specific elastic bands were used to position the sensors on the upper and lower limbs and/or the trunk, depending on the exercise. Based on the sequence of exercises, the patient followed a tutorial that explained, in detail, how to: (i) wear the elastic band around the specified part of the body; (ii) position the sensor inside of the pocket; and (iii) attach the pocket to the elastic band.
Specifically, the instructions specified: (i) which object to use for a certain exercise; (ii) how to position the sensor, and (iii) how to move the object to attain the goal of the games (simulating gestures in daily life). Each patient was supplied with a WeReha device, consisting of tablets and their respective charger, inertial sensor, and charging station and cable; a kit of piston rings; a Velcro pocket, and 3 Smart Objects.
Each device was linked to its specific WeReha server, on which the “web application” was installed; all data was collected each time the patient’s device was connected to the internet. An internet connection was not necessary for proper operation of the device. Whenever the device was connected via Wi-Fi or a cellular network, the data was uploaded to the server.
At the end of each rehabilitation session, the patient or the caregiver was asked to connect the WeReha device to the internet to allow the data to update to the Physiotherapist who could remotely monitor the exercises performed daily. Likewise, the physiotherapist called the patient by phone once a week, with the agreed upon time and day, for a verbal comparison on the progress of physiotherapy with the patient. Upon request by the patient, it was also possible to contact the referred rehabilitation physician if necessary.
First rehabilitative information session
An informative and training session was held by the rehabilitation physician where the patient was recruited to provide him and his caregiver with all of the necessary technical information for correct use of the WeReha device. During the session, the function of the exercises with the biofeedback system (using graphs, objectives, bonuses, or scores) was described, and the physician explained how to understand whether the movement was performed correctly. The caregiver was also instructed on how to help and support the patient when required and to contact the physiotherapist, rehabilitation physician, or bioengineer according if needed. The patient and caregiver were given a pamphlet that provided all of the indications for correct operation of the WeReha at home. The information and first training session had a minimum duration of 30 minutes (up to a maximum of 60 minutes).
The patient underwent a clinical evaluation by the rehabilitation physician and neurologist before starting rehabilitation with the WeReha.
Rehabilitation plan
The rehabilitation process is absorbed through an active rehabilitative week, defined as a week during which the patient performs at least 3 sessions with the WeReha for a minimum of 15 minutes each (the duration is measured by the software), considering the recommended 30 minutes for each session.
The exercises proposed by WeReha took various forms to support the recovery of the hemiplegic side and trunk control. Video-guided exercises with visual and voiced or ringing biofeedback for balance rehabilitation were presented, strengthening the muscles of the legs and arms, and improving the range of motion (ROM) of the joints.
Depending on the exercise, the patient was asked to wear the inertial sensor on the trunk, leg, foot, or wrist detect movements in real time and provide immediate visual feedback to the patient and caregiver. The daily sessions were structured such that patients were to minimize changes in the position of the sensor; to this end, the exercises were grouped into sequences that took this aspect into account. The standing exercises were proposed only in the presence of a caregiver, to prevent falls. During the rehabilitation session, clear indications were provided on how to perform the exercise in written form (on the monitor) and through audio and video tutorials, to create adequate safety conditions and prevent falls.
The WeReha device also included 4 simple objects (a disc, a joystick, a remote control and a spinning top) (see Figure 1), with which the patient could perform specific functional movements for the hand and arm, to improve the articulation of the wrist and grip. Each object had a compartment in which the WeReha inertial sensor could be positioned, thus rendering it “smart.” Through these Smart Objects, patients were able to play simple, interactive, and motivating video games that required them to carry out functional tasks that simulated daily life activities (such as pouring water, rotating an object on a table, moving one’s hand precisely). These games aimed to stimulate the patient's attention by proposing simple solutions to optimize his or her performance for a dual-task training (cognitive-function), which resembles daily tasks. Through the proposal of games with feedback control they focus on not only the recovery of motor function but also on the activation of adequate cognitive strategies (Cognitive-Game Session: Jack the Lumberjack, Drive the Real Hero, Catch the Bear, Switch the Balls, Flowers, Rabbit. Drawbridge, Mine, Monster Hunter, John and the Locker, Colors, Constellation, Potions).
Figure 1.
Smart object, three-dimensional objects printed in plastic material: (a) the disc is used to perform coordinated and specular movements of prono-supination of the wrists, (b) the joystick is used to perform shoulder flexion, elbow flexion-extension and ipsilateral prone-supination movements, (c) the remote control is used to perform elbow flexion-extension, and (d) the spinning top is used to perform a correct grip of the hemiparetic hand and allow the movement of the ipsilateral upper limb.
The patients received points and bonuses and increased their score each time they played a game or performed the exercise: the more correctly the exercise was performed, the higher the scores were. As the points rose, patients received bonuses to increase their motivation and involvement. Each exercise or game had 3 levels of varying difficulties. On the first level, the patient earned 50 points for each correct movement, 100 for the second level, and 150 for the third level. Points accumulated during execution of the exercise, and the final score was shown to the patient at the end of the exercise. To advance to the next level, the patient had to perform the required movement correctly for a certain period or a minimum number of repetitions (established by the team of physicians and physiotherapists for each exercise).
For stability exercises (control of the trunk and balance), the patient was required to stay within a specific range, shown on the tablet as a “target,” while a center of mass, shown on the screen as a “circle,” moved around the “target” following the movements of the patient, as measured by the sensor. For every 0.5 seconds that the patient stayed within the required pause, 50/100/150 points was awarded, depending on the level.
In addition, each time that an exercise was completed, the patient received a “star.” The combination of scores and stars was then shown at the beginning and end of each session. If the patient needed more details, it was always possible to send a more detailed report via e-mail through the web application. In Table 1 is shown an example of rehabilitative program.
Table 1.
Example of rehabilitative session with WeReha.
| N repeat/rest | Upper limb | Lower limb | Balance | Cognitive | |
|---|---|---|---|---|---|
| 1 week | Three sets of 15 repetitions/3 min | Flexion-extension of the shoulder | Knee flexion-extension | Lateral trunk flexions | Game session: Jack the lumberjack |
| Flexion-extension of the elbow | Torsion of the trunk | ||||
| Flexion-extension of the wrist | |||||
| 2 week | Four sets of 15 repetitions/3 min | Flexion-extension of the shoulder | Hip flexion-extension | Lateral trunk flexions | Game sessiona: drive the real hero, catch the bear, switch the balls, flowers, rabbit |
| Flexion-extension of the elbow | Knee flexion-extension | Torsion of the trunk | |||
| Flexion-extension of the wrist | |||||
| Prone-supination of the wrist | |||||
| 3 week | Four sets of 20 repetitions/2 min | Flexion-extension of the shoulder | Hip flexion-extension | Lateral trunk flexions | Game sessiona: drawbridge, mine, monster hunter, john and the locker |
| Abduction adduction of the shoulder | Abduction adduction of the hip | Torsion of the trunk | |||
| Flexion-extension of the elbow | Knee flexion-extension | ||||
| Prone-supination of the wrist | |||||
| 4 week | Four sets of 20 repetitions/1 min | Flexion-extension of the shoulder | Hip flexion-extension | Lateral trunk flexions | Game sessiona: colours, constellation, potions |
| Abduction adduction of the shoulder | Abduction adduction of the hip | Torsion of the trunk | |||
| Flexion-extension of the elbow | Knee flexion-extension | ||||
| Flexion-extension of the wrist | |||||
| Prone-supination of the wrist |
In each basic session the patient performs one of the games listed, or to receive additional prizes he may decide to run more than one game.
Sample Size Calculation
Sample size was calculated starting with preliminary data related to the FM scores of 5 patients with the same inclusion and exclusion criteria, who underwent WeReha rehabilitative treatment with a mean of 101.90 at T0 and 120.05 at T1 with standard deviation of 28.34. For the sample size calculation, the G * Power Version 3.1.9.2 program was used. An (α) of 5% and a power level of 0.90 with a possible dropout of 10%, were considered. The required sample size was 21 patients.
Statistical Analysis
Descriptive statistics were analyzed with respect to the clinical parameters (age, BMI, gender, duration post stroke, affected side, MMSE scale, and education) and the patient rating scales, reporting the mean, median, and standard deviation.
Also, we examined the data by regression analysis for repeated measures using orthogonal polynomial coefficients, using linear and quadratic trends for evaluation times (T0, T1, and T2) of BBS, BI, FM, and mRS scores. Because measurements were made at 3 time points (at T0, T1, and T2), we analyzed the linear and quadratic trends. A linear trend indicated a constant effect of the rehabilitative training for all 3 time points, whereas a quadratic trend indicated that the effect reached a maximum (or minimum) in the middle of the series. For the regression analysis, the polynomial coefficients for the linear trend were −1, 0, and 1, and the coefficients for the quadratic trend were −1, 2, and −1 test was applied to determine whether the linear or quadratic trend was significant enough. Positive (negative) t values for the linear trend indicated a constant increasing (decreasing) effect from T0 to T2 and a maximum (minimum) effect in the middle of the time series.
The Pearson’s r was used to estimate the correlation between TAM scores for dimensions A, B, C, and D. An independent sample t-test for each dimension of the TAM scale was performed according to gender (males vs females).
TAM scores were compared with the rehabilitation assessment scales (Barthel Index, MRS, Fulg-Meyer, and Berg Balance Scale). In particular, we estimated the differences between assessment scale scores at T1 and T0 (training session vs baseline), for which higher positive differences reflected greater positive effects of the WeReha in the training session, and between T2 and T1 (follow-up vs training session), with greater negative differences indicating longer effects of the rehabilitation at the follow-up.
Thus, positive correlations between TAM scores and T1 to T0 values signified that patients with high TAM scores derived the greatest benefit with the method, whereas negative correlations between TAM scores and T2 to T1 values indicated that the benefits lasted longer in the follow-up for patients with high TAM scores. Statistical analyses were performed in R-studio, version 1.2.1335.
Skewness and kurtosis values between −2 and 2 indicated good distribution of the data.42,43 The effect size was estimated in relation to significant t-values and ranged from low <0.5, medium <0.8, and high >0.8.44
Results
Forty-four (N = 44) patients with chronic stroke were recruited, but only 25 patients were included in the study as they met the required criteria; two patients (N = 2) were excluded, because they were noncompliant with the rehabilitative treatment (less than 1session per week or 0 after the first month), and 1 patient (N = 1) withdrew from the study due to medical complications that were not attributable to stroke or to rehabilitative treatment. Consequently, twenty-two (N = 22) patients (mean age 55.34 ± 8.64 years) were analyzed under the following criteria: duration post-stroke (mean 11.5 ± 2.4 months), gender (males N = 15, that is 68% and females N = 7, that is 32%), affected side (right N = 13, that is. 59% and left N = 9, that is 41%), MMSE mean 26 ± 1.8, education (n 1 primary school, n 14 secondary school, and n 7 university) (see Table 2).
Table 2.
Baseline characteristics of participants.
| Population (N = 22) | |
|---|---|
| Gender N (%) | Females 7 (32%); males 15 (68%) |
| Time after stroke, months (mean ± SD) | 11.5 ± 2.4 |
| Affected side N (%) | Right 13 (59%); left 9 (41%) |
| MMSE scale (mean ± SD) | 26 ± 1.8 |
| Education N | One primary school; 14 secondary school; 7 university |
No adverse events or side effects were registered for the WeReha rehabilitative treatment. All the patients enrolled had a referred caregiver (family member or private paid assistant).
Patients reached out for help with approximately of 1.12 ± 2.25 calls per week (often due to difficulties with internet connection).
Also, the patients demonstrated a mean BMI 26.86 ± 3.85; the TAM score reported a mean of 42.45 ± 6.13 for TAM A (perceived ease of use), 35.72 ± 6.47 for TAM B (perceived utility), 22.81 ± 3.97 for TAM C (attitude towards new technologies) and 23.95 ± 5.37 for TAM D (attitude towards the use of new technologies), BI 77.04 ± 14.52, mRS 2.36 ± 1.09, FM 110.40 ± 29.41, BBS 41.45 ± 9.72, at baseline (T0) (see Tables 3 and 4). In regard to the rehabilitation trend assessment, while considering the BI scores, only the quadratic trend was significant (P = .036), and for BBS scores, only the linear trend was significant (P = .008). For the FM and mRS scales, the linear and quadratic trends were both significant (respectively, P = .003linear, P = .021quadratic and P = .047linear, P = .038quadratic) (see Table 5).
Table 3.
Descriptive statistics of age, Body Mass Index (BMI), and Techonology acceptance model (TAM) scores
| Age | BMI | TAM A | TAM B | TAM C | TAM D | |
|---|---|---|---|---|---|---|
| Mean | 55.364 | 26.864 | 42.455 | 35.727 | 22.818 | 23.955 |
| Median | 58.000 | 27.000 | 43.500 | 38.000 | 25.000 | 28.000 |
| Std. deviation | 8.644 | 3.858 | 6.131 | 6.475 | 3.972 | 5.376 |
| Skewness | −0.605 | 0.108 | −0.689 | −0.722 | −1.538 | −1.006 |
| Kurtosis | −0.496 | −0.365 | −0.674 | −0.512 | 1.327 | −0.487 |
TAM A = perceived ease of use; TAM B = perceived utility; TAM C = attitude toward new technologies; TAM D = attitude toward the use of new technologies.
Table 4.
Descriptive statistics of BBS, BI, FM, and mRS scores at baseline (T0), the training session (T1), and follow-up (T2).
| BI |
mRS |
FM |
BBS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | |
| Mean | 77.045 | 80.227 | 79.318 | 2.364 | 1.909 | 1.955 | 110.409 | 129.545 | 133.227 | 41.455 | 43.455 | 44.045 |
| Median | 77.500 | 80.000 | 80.000 | 3.000 | 2.000 | 2.000 | 119.500 | 128.500 | 129.500 | 42.500 | 43.000 | 45.500 |
| Std. deviation | 14.529 | 14.677 | 15.298 | 1.093 | 1.306 | 1.253 | 37.185 | 29.414 | 29.442 | 9.772 | 9.318 | 9.474 |
| Skewness | −0.577 | −0.360 | −0.659 | −0.097 | 0.183 | 0.253 | −0.293 | 0.009 | 0.026 | −1.564 | −2.207 | −2.031 |
| Kurtosis | −0.376 | −0.730 | −0.207 | −1.383 | −1.105 | −1.053 | −0.419 | 0.612 | 0.297 | 4.283 | 7.562 | 6.462 |
Abbreviations: BI, Barthel Index; mRS, Modified Rankin scale; FM, Fulg-Meyer scale; BBS, Berg Balance scale.
Table 5.
Regression analysis of linear and quadratic trends for time series (T0, T1 and T2) of BBS, BI, FM, and mRS scores.
| Scales for rehabilitation assessment | Trend | Estimated coefficient | Std. error | t value | Pr(>|t|) | Cohen’s d | Effect size level |
|---|---|---|---|---|---|---|---|
| BI | lin. | 2.273 | 1.533 | 1.482 | 0.153 | ||
| quadr. | 4.091 | 1.821 | 2.247 | 0.036 | 0.776 | Medium | |
| mRS | lin. | −0.409 | 0.194 | −2.113 | 0.047 | −0.901 | High |
| quadr. | −0.500 | 0.226 | −2.217 | 0.038 | −0.945 | High | |
| FM | lin. | 22.818 | 6.686 | 3.413 | 0.003 | 1.455 | High |
| quadr. | 15.455 | 6.208 | 2.489 | 0.021 | 1.061 | High | |
| BBS | lin. | 2.591 | 0.877 | 2.954 | 0.008 | 1.260 | High |
| quadr. | 1.409 | 0.732 | 1.926 | 0.068 |
Significant P-values are in bold.
Abbreviations: BI, Barthel Index; mRS, Modified Rankin scale; FM, Fulg-Meyer scale; BBS, Berg Balance scale; lin., linear trend; quadr., quadratic trend.
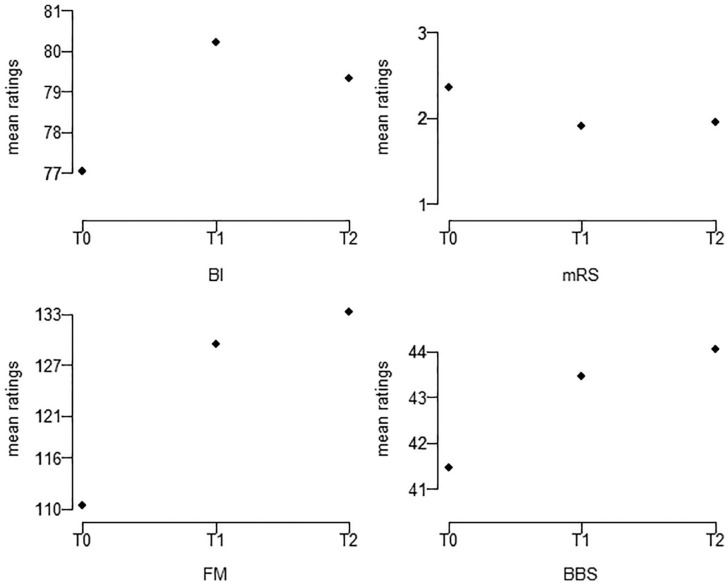
For each scale, the training session scores were higher than at the baseline after WeReha treatment, demonstrating an improvement in the disability function at hand. For the mRS, which measures global disability, the effect was inverted. Follow-up scores remained stable across all scales, except for the BI, which were the scores that had been declined (Figure 2).
Figure 2.
Mean ratings of BBS, BI, FM, and mRS assessment scales in relation to time series (T0 = baseline; T1 = training session; T2 = follow-up).
For Pearson's r correlation, only the correlations between C and A and between B and D were significant with r = 0.473, r = 0.629, r = 0.571 and r = 0.624. Thus, showing that the dimensions of the TAM scale are relatively independent (see Table 6). The subjective ratings displayed that gender had no considerable effect (see Table 7). Considering the correlations between TAM scores for each dimension (A, B, C, and D) and the differences between training session and baseline scores (T1-T0) and between the follow-up and training session scores (T2-T1), the A and C subscales of the TAM correlated significantly only at T2-T1 difference for BI scores with P = .021 and P = .042 (see Table 8).
Table 6.
Correlation between Techonology acceptance model scale (TAM) dimensions.
| TAM A | TAM B | TAM C | TAM D | ||
|---|---|---|---|---|---|
| TAM A | Pearson’s r | − | |||
| P-Value | − | ||||
| TAM B | Pearson’s r | 0.248 | − | ||
| P-Value | 0.266 | − | |||
| TAM C | Pearson’s r | 0.473* | 0.629** | − | |
| P-Value | 0.026 | 0.002 | − | ||
| TAM D | Pearson’s r | 0.099 | 0.571** | 0.624** | − |
| P-Value | 0.661 | 0.005 | 0.002 | − |
TAM A = perceived ease of use; TAM B = perceived utility; TAM C = attitude towards new technologies; TAM D = attitude to the use of new technologies.
P < .05. **P < .01. ***P < .001.
Table 7.
Independent samples t-test for each dimension of the Techonology acceptance model scale ( TAM) in relation to gender (males vs females).
| t values | df | P | |
|---|---|---|---|
| TAM A | −0.649 | 20.000 | .524 |
| TAM B | 0.492 | 20.000 | .628 |
| TAM C | −1.720 | 20.000 | .101* |
| TAM D | −0.614 | 20.000 | .546 |
TAM A = perceived ease of use; TAM B = perceived utility; TAM C = attitude toward new technologies; TAM D = attitude toward the use of new technologies.
Levene’s test is significant (P < .05), suggesting a violation of the equal variance assumption.
Table 8.
Correlations between TAM scores for each dimension (A, B, C, and D) and the differences between training session and baseline scores (T1-T0) and between the follow-up and training session scores (T2-T1) on the assessment scales (BBS, BI, FM, and mRS).
| BI |
mRS |
FM |
BBS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| T1-T0 | T2-T1 | T1-T0 | T2-T1 | T1-T0 | T2-T1 | T1-T0 | T2-T1 | ||
| TAM A | Pearson’s r | 0.037 | –0.490 | 0.222 | −0.017 | 0.050 | 0.174 | 0.335 | −0.250 |
| P-value | 0.871 | 0.021 | 0.320 | 0.942 | 0.826 | 0.439 | 0.128 | 0.262 | |
| TAM B | Pearson’s r | −0.048 | −0.192 | 0.063 | −0.094 | 0.339 | 0.277 | −0.100 | −0.112 |
| P-value | 0.831 | 0.393 | 0.780 | 0.677 | 0.122 | 0.213 | 0.656 | 0.620 | |
| TAM C | Pearson’s r | −0.210 | –0.437 | 0.301 | 0.123 | 0.273 | −0.051 | 0.239 | −0.115 |
| P-value | 0.349 | 0.042 | 0.173 | 0.587 | 0.220 | 0.821 | 0.285 | 0.611 | |
| TAM D | Pearson’s r | 0.004 | −0.014 | 0.115 | 0.168 | 0.268 | 0.077 | 0.284 | 0.034 |
| P-value | 0.986 | 0.950 | 0.609 | 0.455 | 0.229 | 0.732 | 0.200 | 0.882 | |
Significant correlations are in bold. T0 = baseline; T1 = training session; T2 = follow-up; TAM A = perceived ease of use; TAM B = perceived utility; TAM C = attitude toward new technologies; TAM D = attitude toward the use of new technologies.
Abbreviations: BI, Barthel Index; mRS, Modified Rankin scale; FM, Fulg-Meyer scale; BBS, Berg Balance scale.
Discussion
The main aim of our study was to examine the efficacy of the WeReha with respect to the recovery of function in chronic stroke patients and to determine its acceptance by the patients and how it could influence the rehabilitation results.
The TAM score reported in our study shows that the mean of the values for every single sub-scale was high with respect to the limit values. This indicates how, on average, the study sample has positively accepted the technological device. In particular, the TAM-A sub-scale was the one with the highest average value, indicating ease of use perceived by the patient. Furthermore, as shown in the TAM sub-scales A and C, they were significantly related to the T2-T1 difference for BI scores, underlining that there was a linear influence between the patient's perceived ease of use and the predisposition of the patient towards new technologies with improvement in ADL and autonomy. We hypothesize that this was because the device allowed the patient to feel free to perform the rehabilitation session as desired, both in terms of time and place. Also, the study of Chen et al.45 highlighted how devices for telerehabilitation should be designed in order to offer an engaging experience with display of recovery progress and flexibility in schedule and location. Although, Knepley et al.46 showed in their review that there was no consistent evidence to support that telerehabilitation was better than face-to-face rehabilitation, they underlined that it had several advantages, like increased patient satisfaction through interactive video exercises, familiar location and feasibility, allowing telerehabilitation to become a valuable alternative for vulnerable patients.
Our challenge was also to propose a rehabilitation course in chronic patients, in whom the variations in functional recovery are not as evident as in the acute or subacute phase. Often, patients with chronic stroke have difficulty accessing outpatient rehabilitation courses; thus, the possibility for a remote-supervised telerehabilitation course represents one such resource.
The results on the efficacy of the WeReha device for functional recovery were encouraging because they suggested that even in chronic stroke patients (3-24 months after stroke),47 it is possible to experience functional improvements.
BI scores improved significantly after treatment based on their quadratic trend and BBS scores improved as well based on their linear trend. Despite the significance in the results concerning BBS scores, the Minimal Detectable Change (MDC) value of 2.5 to 4.66 points in chronic stroke has not been reached.48
On the other hand, with respect to the FM and mRS scales, the linear and quadratic trends were significant after treatment. Follow-up scores tended to remain stable for all scales, except for the BI, which reported a slight decrease at T2, which was insignificant compared with T1.
The objective of our rehabilitative plan with the WeReha device was to provide post-stroke patients with a rehabilitation solution that could be used easily to execute personalized plans of care in their homes and our results appear to confirm this trend. Moreover, none of the patients reported any accidents while using this rehabilitation device.
Patients could call at any time for help with using the device, and we speculate that this availability ensured good compliance with the rehabilitative treatment, based on the functional improvements that were observed.
The WeReha solution allowed easier supervision of the patient’s progress by the physiotherapist and rehabilitation physician and a more personalized rehabilitative plan of care, fitting the individual needs of each patient.
Home rehabilitation technology solutions have tremendous potential in supporting the short-term and long-term performance of plans of care for motor cognitive rehabilitation in a highly engaging environment, thus empowering the patient, increasing their motivation, positive compliance with the therapy, and improved quality of life.17–19 Such benefits are particularly important for post-stroke patients, especially those in the chronic phase who are typically affected by mobility problems (sometimes associated with different levels of depressions), complicating their travel to clinics in order to attend rehabilitation programs.14
TR can be a suitable alternative to conventional rehabilitation care for post-stroke patients, especially in remote or underserved areas, because many patients have reduced access to care due to limited regional and logistic resources.23 These groups could benefit from a system that allows a health professional to provide rehabilitation services from a remote location.45
Also, patients perceived the WeReha as simple to use and had a positive attitude towards new technologies alike which in turn improved their response to rehabilitation treatments in favor of independence in daily life activities (BI).
TR, combined with other therapies including virtual reality, speech, and robotic assistance, can used as an adjunct to direct in-person care in neurological diseases.49 Increasing and maintaining participation and autonomy in one’s daily routine are promising findings that expand the possibilities for the continuity of rehabilitative care at home using a telemedicine rehabilitative approach.46,50 Moreover, the advantages of TR in comparison to traditional therapy includes greater accessibility to continue rehabilitative care in order to maintain all recovered function and possibly cost reduction for treatments, making telerehabilitation a valid alternative for patients with financial, geographical or transportation constraints.
Furthermore, it allows the specialist and the other members of the multidisciplinary team to be able to stay in contact with their patients easily, check their progress and calibrate their rehabilitation goals.46,50
The WeReha system obtained relevant objective measurements of performance and functional progress in post-stroke patients throughout the rehabilitative therapy, providing feedback and visualizing the data to patients, improving their awareness, empowerment, and motivation, with the support of patient-engaging techniques (gamification techniques).
Limits and strengths
This study is the first to experiment with the effects of the WeReha device in the rehabilitation of chronic stroke patients. Among the ability of this device to improve rehabilitative results, as described above, certainly an important aspect is to promote its adherence to home exercise.
However, this study has several limitations. We did not have the opportunity to report the FM scale as divided into the different components, so the results presented reflect a global improvement in disabilities that cannot be traced back to the upper or lower limbs specifically. Furthermore, the TAM scale was reported only to the patient and not to the caregivers or clinical professionals. Therefore, future studies would be desirable to investigate this topic further. The limitations connected to the lack of a control group could also be considered as bias.
Conclusion
The goal of this study was to examine the applicability and value of an innovative technology product, such as the WeReha, in the home-based rehabilitation of stroke patients as an integrative solution to a conventional exercise program and assess its acceptance by the patient. Our results are encouraging and demonstrate that the WeReha is readily adaptable, allowing only those who are authorized to assign exercises, by planning specific sequences of movements that are supervised remotely by the physiotherapist. The WeReha home rehabilitative device could be a feasible and efficient integrative solution to a conventional exercise program for stroke patients. Future research should expand these data in randomized controlled trials.
Acknowledgments
We are grateful to Enrica Di Sipio, Quality Manager and Product Specialist of CoRehab (Italy) and Product Manager in the Magic PCP European Project.
Footnotes
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Magic project had a grant from the European Union, which concerns the clinical experience of the “G. D’Annunzio ”University of Chieti (Italy)
Declaration of conflicting interests:The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors state that no financial or personal interests have influenced this work. This study is part of the Magic-PCP Project [NCT03964662], with a grant from the European Union, which concerns the clinical experience of the “G. D’Annunzio” University and IRCSS “Bonino Pulejo”.
Author Contributions: RGB and RS responsible for the project, conceptualization and draft; LP, AB and VC data collection, AS and MT statistical analysis, TP data interpretation, writing and manuscript draft.
ORCID iD: L Pezzi  https://orcid.org/0000-0002-4816-9126
https://orcid.org/0000-0002-4816-9126
References
- 1. Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke. 2017;12:13-32. doi: 10.1177/1747493016676285 [DOI] [PubMed] [Google Scholar]
- 2. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017-1025. doi: 10.1111/ijs.12357 [DOI] [PubMed] [Google Scholar]
- 3. Toglia J, Askin G, Gerber LM, Jaywant A, O’Dell MW. Participation in younger and older adults post-stroke: frequency, importance, and desirability of engagement in activities. Front Neurol. 2019;10:1108. doi: 10.3389/fneur.2019.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. J Am Med Assoc. 2018;319:820-821. [DOI] [PubMed] [Google Scholar]
- 5. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98-e169. Published online May 4, 2016. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 6. Kiper P, Szczudlik A, Agostini M, et al. Virtual reality for upper limb rehabilitation in subacute and chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2018;99:834-842.e4. [DOI] [PubMed] [Google Scholar]
- 7. Kristensen HK, Tistad M, von Koch L, et al. The importance of patient involvement in stroke rehabilitation. PLoS One. 2016;11:e0157149. doi: 10.1371/journal.pone.0157149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodgers H, Price C. Stroke unit care, inpatient rehabilitation and early supported discharge. Clin Med (Lond). 2017;17:173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langhorne P, Baylan S, Trialists ESD. Early supported discharge services for people with acute stroke. Cochrane Database Syst Rev. 2017;7:CD000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chumbler NR, Quigley P, Li X, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomized, controlled trial. Stroke. 2012;43:2168-2174. doi: 10.1161/STROKEAHA.111.646943 [DOI] [PubMed] [Google Scholar]
- 11. Burdea GC, Grampurohit N, Kim N, et al. Feasibility of integrative games and novel therapeutic game controller for telerehabilitation of individuals chronic post-stroke living in the community. Top Stroke Rehabil. 2019;27:321-336. Published online December 25, 2019. doi: 10.1080/10749357.2019.1701178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caetano LCG, Pacheco BD, Samora GAR, et al. Self-efficacy to engage in physical exercise and walking ability best predicted exercise adherence after stroke. Stroke Res Treat. 2020;2020:2957623. doi: 10.1155/2020/2957623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee HC, Huang CL, Ho SH, et al. The effect of a virtual reality game intervention on balance for patients with stroke: a randomized controlled trial. Games Health J. 2017;6:303-311. Published online August 3, 2017. doi: 10.1089/g4h.2016.0109 [DOI] [PubMed] [Google Scholar]
- 14. Cheng D, Qu Z, Huang J, et al. Motivational interviewing for improving recovery after stroke. Cochrane Database Syst Rev. 2015;2015:CD011398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald SG, Cooper RA, Thorman T, et al. The GAMECycle exercise system: comparison with standard ergometry. J Spinal Cord Med. 2004;27:453-459. [DOI] [PubMed] [Google Scholar]
- 16. Bernocchi P, Vanoglio F, Baratti D, et al. Home-based telesurveillance and rehabilitation after stroke: a real-life study. Top Stroke Rehabil. 2016;23:106-115. Published online January 9, 2016. doi: 10.1080/10749357.2015.1120453 [DOI] [PubMed] [Google Scholar]
- 17. da Silva Ribeiro NM, Ferraz DD, Pedreira É, et al. Virtual rehabilitation via Nintendo Wii® and conventional physical therapy effectively treat post-stroke hemiparetic patients. Top Stroke Rehabil. 2015;22:299-305. Published online February 25, 2015. doi: 10.1179/1074935714Z.0000000017 [DOI] [PubMed] [Google Scholar]
- 18. Maier M, Rubio Ballester B, Duff A, et al. Effect of specific over nonspecific VR-based rehabilitation on poststroke motor recovery: a systematic meta-analysis. Neurorehabil Neural Repair. 2019;33:112-129. Published online January 20, 2019. doi: 10.1177/1545968318820169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellington A, Adams R, White M, et al. Behavioral intention to use a virtual instrumental activities of daily living system among people with stroke. Am J Occup Ther. 2015;69:6903290030p1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynch EA, Jones TM, Simpson DB, et al. Activity monitors for increasing physical activity in adult stroke survivors. Cochrane Database Syst Rev. 2018;7:CD012543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497-1500. [DOI] [PubMed] [Google Scholar]
- 22. Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014;2014:CD010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarfo FS, Ulasavets U, Opare-Sem OK, et al. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis. 2018;27:2306-2318. Published online June 4, 2018. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tchero H, Tabue-Teguo M, Lannuzel A, et al. Telerehabilitation for stroke survivors: systematic review and meta-analysis. J Med Internet Res. 2018;20:e10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appleby E, Gill ST, Hayes LK, et al. Effectiveness of telerehabilitation in the management of adults with stroke: a systematic review. PLoS One. 2019;14:e0225150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096. Published online February 1, 2007. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 27. Mancuso M, Demeyere N, Abbruzzese L, et al. Using the Oxford cognitive screen to detect cognitive impairment in stroke patients: a comparison with the mini-mental state examination. Front Neurol. 2018;9:101. doi: 10.3389/fneur.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham JE, Karmarkar AM, Ottenbacher KJ. Small sample research designs for evidence-based rehabilitation: issues and methods. Arch Phys Med Rehabil. 2012;93:S111-S116. Published online May 8, 2012. doi: 10.1016/j.apmr.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Des Jarlais DC, Lyles C, Crepaz N, TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berg KO, Maki BE, Williams JI, et al. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73:1073-1080. [PubMed] [Google Scholar]
- 31. Pollock AS, Durward BR, Rowe PJ, et al. What is balance? Clin Rehabil. 2000;14:402-406. [DOI] [PubMed] [Google Scholar]
- 32. Tyson SF, DeSouza LH. A clinical model for the assessment of posture and balance in people with stroke. Disabil Rehabil. 2003;25:120-126. [DOI] [PubMed] [Google Scholar]
- 33. Castiglia SF, Galeoto G, Lauta A, et al. The culturally adapted Italian version of the Barthel Index (IcaBI): assessment of structural validity, inter-rater reliability and responsiveness to clinically relevant improvements in patients admitted to inpatient rehabilitation centers. Funct Neurol. 2017;22:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703-709. [DOI] [PubMed] [Google Scholar]
- 35. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232-240. [DOI] [PubMed] [Google Scholar]
- 36. Turolla A, Dam M, Ventura L, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. J Neuroeng Rehabil. 2013;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. 2017;48:2007-2012. Published online June 16, 2016. doi: 10.1161/STROKEAHA.117.017866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis FD, Bagozzi RP, Warshaw PR. User acceptance of computer technology: a comparison of two theoretical models. Manag Sci. 1989;35:982-1003. [Google Scholar]
- 39. Rahimi B, Nadri H, Lotfnezhad Afshar H, et al. A systematic review of the technology acceptance model in health informatics. Appl Clin Inform. 2018;9:604-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cikajlo I, Rudolf M, Mainetti R, et al. Multi-exergames to set targets and supplement the intensified conventional balance training in patients with stroke: a randomized pilot trial. Front Psychol. 2020;11:572. doi: 10.3389/fpsyg.2020.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esteki-Ghashghaei F, Saadatnia M, Khorvash F, et al. The effect of home base physical activity program based on the BASNEF model on motor recovery in patients with stroke. Home Health Care Serv Q. 2020;39:1-14. Published online May 13, 2020. doi: 10.1080/01621424.2020.1765938 [DOI] [PubMed] [Google Scholar]
- 42. Gravetter F, Wallnau L. Essentials of statistics for the behavioral sciences. 8th ed. Wadsworth; 2014. [Google Scholar]
- 43. Trochim WM, Donnelly JP. The research methods knowledge base. 3rd ed. Atomic Dog; 2006. [Google Scholar]
- 44. Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Chen Y, Zheng K, et al. A qualitative study on user acceptance of a home-based stroke telerehabilitation system. Top Stroke Rehabil. 2020;27:81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Knepley KD, Mao JZ, Wieczorek P, et al. Impact of telerehabilitation for stroke-related deficits. Telemed J E Health. Published online April 23, 2020. doi: 10.1089/tmj.2020.0019 [DOI] [PubMed] [Google Scholar]
- 47. Dodakian L, McKenzie AL, Le V, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. 2012;93:1201-1208. Published online April 12, 2012. doi: 10.1016/j.apmr.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 49. Holden MK, Dyar TA, Dayan-Cimadoro L. Telerehabilitation using a virtual environment improves upper extremity function in patients with stroke. IEEE Trans Neural Syst Rehabil Eng. 2007;15:36-42. [DOI] [PubMed] [Google Scholar]
- 50. Isernia S, Pagliari C, Jonsdottir J, et al. Efficiency and patient-reported outcome measures from clinic to home: the human empowerment aging and disability program for digital-health rehabilitation. Front Neurol. 2019;10:1206 Published November 19, 2019. doi: 10.3389/fneur.2019.01206 [DOI] [PMC free article] [PubMed] [Google Scholar]