Abstract
Background
The number of people living with stroke has increased demand for rehabilitation. A potential solution is telerehabilitation for health care delivery to promote self-management. One such approach is the Augmented Community Telerehabilitation Intervention (ACTIV). This structured 6-month program uses limited face-to-face sessions, telephone contact, and text messages to augment stroke rehabilitation.
Objective
To investigate whether ACTIV improved physical function compared with usual care.
Methods
This 2-arm, parallel randomized controlled trial was conducted in 4 New Zealand centers. Inclusion criteria were patients with first-ever stroke, age >20 years, and discharged home. A blinded assessor completed outcome measurement in participants’ homes at baseline, postintervention, and 6 months postintervention. Stratified block randomization occurred after baseline assessment, with participants allocated to ACTIV or usual care control.
Results
A total of 95 people were recruited (ACTIV: n = 47; control: n = 48). Postintervention intention-to-treat analysis found a nonsignificant difference between the groups in scores (4·51; P = .07) for physical function (measured by the physical subcomponent of the Stroke Impact Scale). The planned per-protocol analysis (ACTIV: n = 43; control: n = 48) found a significant difference in physical function between the groups (5·28; P = .04). Improvements in physical function were not maintained at the 12-month follow-up.
Conclusions
ACTIV was not effective in improving physical function in the ACTIV group compared with the usual care group. The per-protocol analysis raises the possibility that for those who receive more than 50% of the intervention, ACTIV may be effective in preventing deterioration or even improving physical function in people with stroke, in the period immediately following discharge from hospital.
Keywords: telerehabilitation, stroke, randomized controlled trial
Introduction
International guidelines recommend that stroke rehabilitation should continue until agreed goals are reached, with regular reassessment to ensure gains are maintained.1-3 Unfortunately, limited health budgets mean that this is unattainable for most publicly funded health services. Using standard mobile phones to deliver rehabilitation offers a potential solution. Mobile phones have been used to support people with coronary heart disease to modify risk4 and to increase physical activity in sedentary adults.5 However, the effectiveness of using mobile phones to deliver stroke rehabilitation to improve physical function and reduce the sedentary behavior that frequently occurs after hospital discharge6 has not been investigated.
The Augmented Community Telerehabilitation Intervention (ACTIV) is a structured 6-month program developed by a team of clinicians and researchers in neurological rehabilitation.7 The program uses a combination of face-to-face sessions, telephone contact, and text message reminders to support ongoing physical activity. The intervention was developed based on clinical expertise, the findings from a feasibility and acceptability trial,8 and theoretical principles drawn from Bandura’s theory of behavior change.9 A core tenet of Bandura’s theory is that people are motivated by anticipation of achievement and self-efficacy, which is defined as a person’s belief in their ability to effect change. Bandura asserted that people are proactive and aspirational, so planning to achieve challenging goals increases effort. In turn, this helps people persist in activities and leads to behavior change. The objective of the present study was to determine if ACTIV improved physical function for people with stroke compared with a usual care control group. Secondary objectives were the maintenance of any gains at 12 months and changes in physical performance, stroke self-efficacy, health outcomes, and quality of life.
Methods
Trial Design
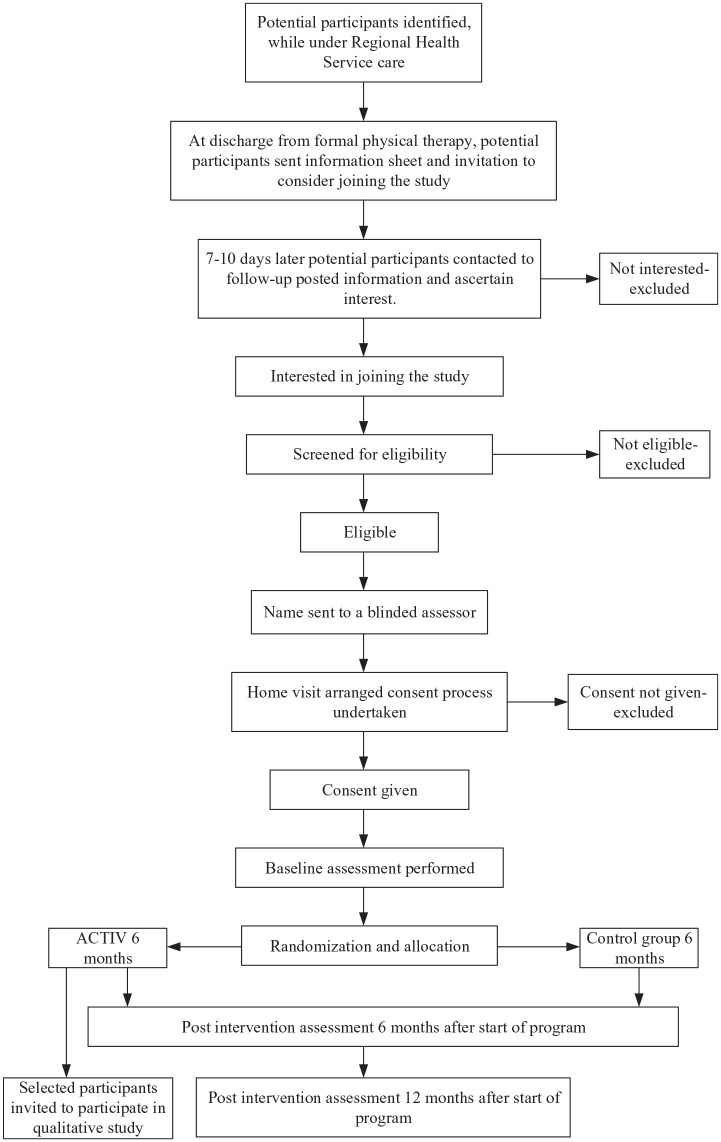
The ACTIV trial was a 2-arm, assessor-blinded, parallel randomized controlled trial. Details of the trial design can be found in the protocol.7 Participant enrolment occurred in 4 centers across New Zealand. People were eligible for trial inclusion at the time of discharge from inpatient or community-based rehabilitation. Figure 1 shows participant progress through the trial.
Figure 1.
Participation progress through the study.
Abbreviations: ACTIV, Augmented Community Telerehabilitation Intervention.
Trial Setting
All assessment of outcome measures and delivery of the ACTIV intervention took place in participants’ own homes or remotely via telephone contact and text messages.
Recruitment
Participants were recruited through publicly funded District Health Board inpatient and community stroke rehabilitation services. An information sheet about the trial and an invitation letter were mailed to all patients who met the inclusion criteria. The inclusion criteria were as follows: adults aged >20 years who had experienced a first-ever hemispheric stroke of hemorrhagic or ischemic origin and were discharged from inpatient, outpatient, or community physiotherapy services to live in their own home. Exclusion criteria were a confirmed brain stem or cerebellar stroke or inability to understand and speak basic-level English.
Potential participants received telephone screening to determine eligibility 7 to 10 days after receiving the trial information. Those who were not able to be contacted were telephoned on at least 7 occasions, on different days, at different times of the day, over a 2-week period before being excluded from the trial. To be included, participants needed to score at least 3 on a telephone-administered cognitive screening questionnaire,10 have a limitation in physical function (leg, arm, or both), and have had medical clearance from their general practitioner11 to participate in a low- to moderate-level activity program. Limitation in the physical function of the leg was established if participants scored between 3 and 5 on the Functional Ambulation Category.12 If they received a score of 5, participants were required to have experienced a reduction in walking capacity, either speed or distance. Limitation in arm function was established using the questions below. Participants needed to have some arm function (answering yes to at least 1 question in section A), but some impairment (answering no to at least 1 question in section B).
- A. With your affected arm are you able to
- switch on a light?
- bring a glass of water to your mouth?
- move your fingers and thumb independently?
- B. Are you able to
- use a keyboard equally with both hands?
- holding a pencil with your affected hand, make rapid dots on a piece of paper?
- take a spoonful of liquid to your mouth without spilling it or bending your neck?
Potential participants who met the eligibility criteria and consented to entering the trial were referred to an assessor for baseline evaluation. The assessor contacted them, made an appointment for the evaluation, and rechecked consent at the first face-to-face encounter. Ethical approval was obtained for a minor change to the trial design in May 2013. This was because some potential participants had insufficient English themselves but had supportive family who wished to translate.
Randomization and Blinding
Randomization occurred after the baseline assessment. Participants were allocated to an ACTIV group or a usual care control group using a 1:1 ratio. Stratified block randomization was used according to participants’ geographic center and baseline mobility. Random block sizes were used that ensured a probability smaller than 0.1% that balance would be broken across strata by 4 or more participants. The randomization software was coded and tested by the trial statistician, then handed to an independent party for random number generator seeding, execution of allocation, and day-to-day management of randomization. The recruiters, assessors, and personnel involved in data management and analysis were blinded to participants’ treatment allocation. Participants were contacted by the blinded assessor for baseline assessment, and randomization occurred within 2 days. The independent party then informed the intervention physical therapist which participants were allocated to the ACTIV group, and these participants were contacted for a suitable time to start ACTIV. A research assistant contacted participants randomized to the control arm to inform them of their group allocation.
Intervention: ACTIV
ACTIV focused on 2 functional categories: “staying upright” and “using your arm.” There were standard exercises for each category. Each exercise had parameters that could be selected and modified, allowing the program to be tailored to individuals. Further details of the program can be found in Supplemental material 2. The program was delivered by physical therapists who had completed ACTIV training, (Supplemental material 1, Table II). The physical therapists established patient-centered goals at the first home visit. Next, they selected exercises and activities to address these goals, accessing advice from an expert neurological physical therapist if required. Each participant received 4 face-to face visits, 5 structured phone calls, and personalized text messages (Supplemental material 1, Figure I). The phone calls focused on helping participants formulate a strategy to maximize their engagement in the program. For example, the physical therapist had a copy of the participant’s exercise chart, so they knew the participant’s current exercise plan and were able to address any reported difficulty with exercise completion. They could also clarify exercise instructions or implement a change in the level of challenge by altering exercise parameters. Text messages were used to encourage continuation of exercises and acknowledge participants’ progress (Supplemental material 1, Table I).
Usual Care Control Group
Standard care following discharge from rehabilitation services in New Zealand usually means no further formal rehabilitation. To ensure usual care, no attempt was made to discourage any additional care, and this was not measured.
Data Monitoring
The progress of the trial was monitored by a Data Monitoring Committee, which comprised the trial statistician and 3 researchers independent of this trial.
Outcome and Other Measures
All assessments took place in participants’ homes. A blinded assessor collected outcome measures at baseline. The second assessment took place 6 months after randomization (at the end of the intervention), and the third occurred 12 months after randomization.
Baseline Data
Baseline demographic data were collected from all participants.
Primary Outcome Measure
The primary outcome measure was the physical subcomponent of the Stroke Impact Scale (SIS3.0),13 with the primary end point at 6 months. This measure sums the scores from the 4 physical domains (strength, hand function, mobility, and activities of daily living) and is valid as a stand-alone measure. Rasch analysis indicated that the domains were unidimensional and investigated a range of physical functions that people with stroke find difficult.14 The SIS3.0 result is reported as a normalized summary score calculated from the SIS database. The SIS has been shown to be responsive to change.15
Secondary Outcome Measures
Physical performance measures
Hand grip strength and balance were also measured. A JAMAR hand-held dynamometer (Sammons Preston, Rolyan, Bolingbrook, IL) was used to measure hand grip strength.16 Balance was assessed using the Step Test, which requires repetitive stepping on and off a 7.5-cm step while remaining in a single-leg stance on the test leg.17 These 2 measures were also selected because of their ease and speed of administration.
Self-efficacy
The Stroke Self-Efficacy Questionnaire (SSEQ) was used to collect data relating to participants’ confidence in their ability to undertake daily tasks. The SSEQ has been shown to have good face validity, and a criterion validity of 0.80 when compared with the Falls Efficacy Scale.18
Health outcomes and the impact of stroke
The overall stroke recovery rating of the SIS3.013 as well as each of the 8 domains were used to ascertain changes in health outcomes and the impact of stroke on participants’ lives. The test-retest reliability for all SIS domains is excellent (interclass correlation coefficient [ICC] > 0.90), except for the emotion domain, which had an ICC of 0.68.19 The Visual Analogue Scale (VAS) of the EuroQol 5D (EQ-5D) allowed participants to rate their health from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”). The EQ-5D VAS has acceptable test-retest reliability, with ICC values from 0.67 to 0.81 in people with stroke.20
Adverse Events
A research assistant blinded to group allocation telephoned each participant on a monthly basis to record adverse events. Physical activity outside the study (PAOS) was also recorded to ascertain potential relatedness of any adverse events. Adverse events were coded by 2 independent assessors, also blinded to group allocation, according to the Common Terminology Criteria for Adverse Events version 4.0.21
Data Analysis
The primary analyses were conducted using an intention-to-treat analysis set, comprising participants with nonmissing outcome data for the efficacy analyses and all participants for the sensitivity analyses, assigned to the trial arm to which they were randomized. Under an assumption of missingness at random, a covariate-adjusted analysis of complete data is a reasonable approach when sensitivity analyses involving all participants are performed.22 A per-protocol set was also predefined, in which participants in the ACTIV arm who withdrew before receiving 50% of the intervention (2 visits, 3 phone calls, and half of the text messages) were excluded from analysis unless all their goals had been met on withdrawal. The difference in the primary outcome at 6 months between the 2 groups was determined using a linear mixed model adjusted for baseline and other covariates, which were selected following a blind review for each outcome from among a core set of prespecified variables. Covariates were retained for adjustment purposes based on their partial R2 in the presence of all covariates from the core set but in the absence of treatment effect. The model accounted for recruitment center using random effects.
Secondary analyses were performed to examine all efficacy outcomes over time in a mixed-effects model with center-associated random effects. These models were linear except for admissions data, which were analyzed using mixed logistic regression and Step Test data, which were analyzed using a zero-inflated negative binomial distribution with logarithmic link based on the blind review. Multiple imputation was used for missing baseline data using the full conditional specification of all baseline covariates (including outcomes measured at baseline; Supplemental material 1, Table III). Primary and secondary outcomes were analyzed in the intention-to-treat analysis set. Secondary analyses were conducted using the per-protocol analysis set to assess the effect of adherence to the intervention.
Sample Size Calculation
The clinically important difference for the primary outcome was established at 5.36 using the truncated geometric mean of the clinically important differences for the 4 relevant SIS3.0 domains, as reported by Lin et al.13 Using variance estimates from a rehabilitation trial by Marsden et al,23 38·4 participants per arm were required to detect a clinically important difference with 80% power using a linear model. We conservatively assumed no increase in efficiency from an adjustment for baseline value or other covariates. A total sample size of 96 participants was required, allowing for an attrition rate of 20% over the 12-month research period. This meant that the probability was approximately 80% that the trial would detect a treatment difference if the between-group difference was at least 5.4 points in the SIS3.03 physical subcomponent.
Results
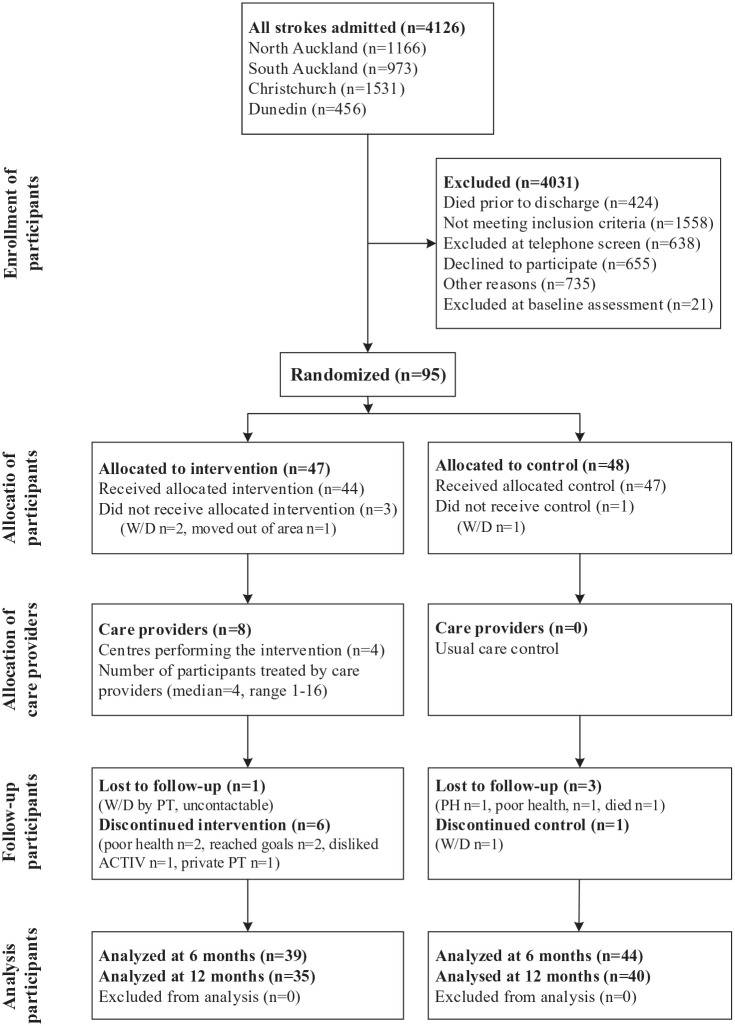
Participants were recruited from 4 centers across New Zealand between August 2012 and May 2015. In total, 4126 patients were admitted to these centers with a diagnosis of stroke. Early identification of people with stroke ensured that no potential participant was missed. Patients were excluded if they died before discharge (n = 424) or did not meet the inclusion criteria for this study: subsequently diagnosed as not first stroke (n = 542), not discharged home (n = 644), and brainstem or cerebellar stroke (n = 372). Other reasons for exclusion were declined to participate (n = 655), unable to be contacted (n = 428), and taking part in other research (n = 307). The remainder of the excluded patients (n = 659) did not meet the physical or cognitive inclusion criteria. A total of 95 eligible patients agreed to participate and were randomized to the trial. Table 1 presents participants’ demographic data, and Figure 2 shows the CONSORT diagram.
Table 1.
Baseline Data for Included Participants.a
| ACTIV, n = 47 | Control, n = 48 | ||
|---|---|---|---|
| Age, years | Mean (SD) | 74.1 (11.7) | 72.9 (11.7) |
| Sex | Male | 23 | 26 |
| Female | 24 | 22 | |
| Stroke side | Right | 22 | 26 |
| Left | 25 | 22 | |
| Stroke type | Ischemic | 40 | 45 |
| Hemorrhagic | 7 | 3 | |
| Time since stroke, months | Mean (SD) | 7.2 (3.3) | 6.1 (2.8) |
| Center | North Auckland | 18 | 19 |
| South Auckland | 7 | 7 | |
| Christchurch | 16 | 14 | |
| Dunedin | 6 | 8 | |
| Living situation | Accompanied | 34 | 35 |
| Alone | 13 | 11 | |
| Missing | 0 | 2 | |
| Ethnic group | European | 43 | 43 |
| Non-European | 3 | 4 | |
| New Zealand Māori | 1 | 1 | |
| Mobility level | More mobile | 23 | 21 |
| Less mobile | 24 | 27 | |
| Depression | Moderate depression | 20 | 22 |
| No depression | 27 | 26 |
Abbreviation: ACTIV, Augmented Community Telerehabilitation Intervention.
Mobility level based on Functional Ambulation Category: more mobile = 5; less mobile = 3 or 4.
Figure 2.
CONSORT diagram.
Abbreviations: ACTIV, Augmented Community Telerehabilitation Intervention; PH, moved to live in a private hospital; private PT, selected to access private physical therapy instead of ACTIV; W/D: withdrawn.
Multiple imputation only affected 2 baseline EQ-5D values and 2 baseline living situation values and was, therefore, involved in the analysis of EQ-5D and SIS strength and emotional domain scores. The relative increase in variance caused by multiple imputation was <0.2% in all cases.
Primary Outcome: SIS Physical Subcomponent
In the intention-to-treat analysis, the effect of ACTIV on the primary outcome measure at 6 months did not reach significance (4.51; 95% CI = −0.46, 9.48; P = .07). Table 2 and Supplemental material 1, Table IV show results of the intention-to-treat analysis.
Table 2.
Outcomes at the Primary End Point (After Intervention).
| ACTIV |
Control |
Adjusted difference (95% CI) 6 months |
P value |
||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | ||||
| SIS3.0 Physical | Mean (SD) | 69.4 (16.0) | 72.5 (15.8) | 63.3 (19.4) | 64.4 (18.8) | 4·51 (−0·46, 9·48) | .07 |
| n | 47 | 39 | 48 | 44 | |||
| Grip strength (affected) | Mean (SD) | 14.4 (9.2) | 16.3 (9.3) | 16.7 (10.4) | 18.5 (10.5) | −0·29 (−2·32, 1·73) | .77 |
| n | 47 | 39 | 48 | 44 | |||
| Grip strength (unaffected) | Mean (SD) | 24.0 (12.0) | 25.2 (11.5) | 27.2 (14.3) | 28.5 (13.3) | 0·20 (−1·56, 1·96) | .82 |
| n | 47 | 39 | 48 | 44 | |||
| Step number (affected)a | Mean (SD) | 7.4 (4.5) | 7.9 (4.9) | 7.1 (5.4) | 7.4 (6.1) | 0·06 (−0·11, 0·23) | .50 |
| n | 47 | 39 | 48 | 42 | |||
| Step number (unaffected)a | Mean (SD) | 8.2 (5.1) | 8.5 (5.2) | 8.0 (5.3) | 8.5 (5.9) | −0·02 (−0·18, 0·14) | .79 |
| n | 47 | 39 | 48 | 42 | |||
| SSEQ | Mean (SD) | 99.9 (20.1) | 105.5 (19.9) | 90.7 (30.9) | 93.9 (28.3) | 6·15 (−1·37, 13·67) | .11 |
| n | 47 | 39 | 48 | 44 | |||
| SIS | Mean (SD) | 58.5 (19.5) | 67.3 (21.3) | 53.5 (20.1) | 61.8 (19.6) | 2·68 (−5·35, 10·70) | .51 |
| n | 47 | 39 | 48 | 44 | |||
| EQ-5D VAS | Mean (SD) | 69.9 (18.0) | 76.2 (17.8) | 60.3 (19.7) | 62.4 (25.7) | 10·09 (0·53, 19·65) | .04b |
| n | 45 | 38 | 48 | 41 | |||
| SIS3.0: Strength | Mean (SD) | 64.1 (16.9) | 65.4 (19.8) | 54.0 (19.5) | 54.3 (18.7) | 4·63 (−2·11, 11·38) | .18 |
| n | 47 | 39 | 48 | 44 | |||
| SIS3.0: Memory | Mean (SD) | 74.6 (17.5) | 80.6 (14.9) | 72.7 (19.9) | 73.7 (23.9) | 4.43 (−1.11, 9.97) | .12 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: Emotion | Mean (SD) | 75.8 (15.0) | 77.0 (16.9) | 70.4 (18.8) | 70.3 (21.7) | 4.59 (−1.44, 10.62) | .13 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: Communication | Mean (SD) | 81.7 (16.7) | 84.9 (16.3) | 80.6 (20.1) | 82.7 (18.6) | 1.88 (−3.76, 7.52) | .51 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: ADL | Mean (SD) | 72.8 (16.1) | 75.4 (17.4) | 69.4 (20.0) | 70.5 (22.5) | 5.26 (−0.50, 11.02) | .07 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: Mobility | Mean (SD) | 71.8 (17.8) | 74.6 (17.0) | 66.9 (21.7) | 61.7 (25.0) | 2.67 (−3.06, 8.40) | .36 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: Use of hand | Mean (SD) | 62.7 (31.9) | 68.8 (26.9) | 58.1 (29.9) | 58.3 (31.9) | 6.43 (−2.37, 15.22) | .15 |
| n | 47 | 39 | 44 | 40 | |||
| SIS3.0: Participation | Mean (SD) | 62.1 (21.3) | 72.4 (22.0) | 57.9 (24.8) | 61.3 (24.1) | 11.34 (2.54, 20.14) | .01b |
| n | 47 | 39 | 44 | 40 | |||
Abbreviations: ACTIV, Augmented Community Telerehabilitation Intervention; ADL, activities of daily living; EQ-5D VAS, Visual Analogue Scale to measure health status; n, number in analysis set; SIS, Stroke Impact Scale; SSEQ, Stroke Self-Efficacy Questionnaire.
Natural logarithmic scale.
Statistically significant at 5% level.
The per-protocol analysis set excluded 4 participants, 3 of whom had no outcome data at 6 months. The effect of ACTIV on the primary outcome based on the per-protocol analysis was significant (4.98; 95% CI = 0.003, 9.95; P = .0499) and indicated that ACTIV improved physical function after stroke for those who received at least 50% of the intervention (Supplemental material 1, Table V).
Secondary Outcomes
At the 12-month follow-up, the effect of ACTIV on the SIS3.0 physical function subscale was nonsignificant (1.72; 95% CI = −4.04, 7.48; P = .55), suggesting that there was no retention of gains made during the intervention.
At the end of the intervention (6-month assessment), the effect for the participation subscale of the SIS showed a significant beneficial effect in favor of ACTIV (11.34; 95% CI = 2.54, 20.14; P = .012). However, this was not sustained at the 12-month assessment because there were no between-group differences in any SIS3.0 domains. In addition, ACTIV showed no significant effects on grip strength, balance, or self-efficacy (SSEQ).
The ACTIV group showed a significant improvement on the EQ-5D VAS at 6 months (10.09; 95% CI = 0.53, 19.65; P = .04). The effect of ACTIV on the EQ-5D VAS at 12 months was also significant, but participants in the intervention group had significantly lower EQ-5D VAS scores than those in the control group (−10.76; 95% CI = −19.86, −1.67; P = .02). Intervention fidelity was planned (Supplemental material 1, Table VI) and measured (Supplemental material 1, Tables VII, VIII, IX, and X). The results of the sensitivity analyses (Supplemental material 1, Figure II) showed that the best and worst cases for the intervention lay considerably outside the confidence bounds for grip strength outcomes and, to a lesser extent, the SIS physical subcomponent, SSEQ, and SIS3.0 stroke recovery rating scores. Results from the intention-to-treat extension and return-to-baseline analyses clustered close to the efficacy estimates in all cases.
Adverse Events
Adverse events were assessed for severity and relatedness to the intervention.21 Information collected on PAOS was used to help adjudicate relatedness. There was 1 death in the control group but no severe injuries in either group. The number of participants who undertook POAS was 26 in the intervention and 27 in the control group. There were several mild to moderate events that did not require medical intervention (ACTIV: n = 28; control: n = 25); however, only one of these events was attributed to the intervention (mild exacerbation of osteoarthritis). There were no significant differences between the groups in the incidence of adverse effects at any severity level (P = .47).
Discussion
Based on the findings from the intention-to-treat analysis, participants in the intervention group did not show significant changes in physical function at the end of the intervention period compared with the usual-care control group. All participants had already completed usual rehabilitation, meaning it was not clear at the outset of the trial whether a small addition of input would affect physical function. The intervention group received 14 hours of contact over 6 months, of which 9 hours were delivered remotely; this was insufficient to effect a significant change in physical activity. However, based on the planned per-protocol analysis, participants who received at least 2 visits, 3 phone calls, and half of the text messages demonstrated significant improvements in physical function, participation, and quality of life. These improvements were measured at the conclusion of the intervention (6-month assessment); however, there was no retention of improvements once the contact was withdrawn. The results from the ACTIV trial demonstrated that using ubiquitous technology to augment minimal face-to-face contacts can improve physical function or prevent the decline in mobility found after discharge from rehabilitation.24 Recent systematic reviews suggested that telerehabilitation may increase access to services for people in underresourced areas and noted that mobile phones are a feasible, low-cost rehabilitation delivery method.25,26 The most recent Cochrane review that investigated telerehabilitation after stroke27 highlighted the paucity of adequately powered trials; ACTIV addressed this limitation.
Participation is a construct that appears resistant to change and is not always a natural consequence of functional improvements.28 ACTIV emphasized participant-selected, valued activities as goals, which led to a significant improvement in the SIS3.0 participation domain at the end of the intervention period; however, these gains were not maintained 6 months after the intervention ended. The EQ-5D at the 12-month follow-up showed that participants felt that their health was worse than at baseline. People who had experienced support via ACTIV followed by its withdrawal might have deferred the disappointment experienced at discharge from rehabilitation to discharge from ACTIV. The reduction in hope is consistent with research in the area of rehabilitation and recovery.29 For many people, discharge from rehabilitation implies the end of further improvement.
The structure of ACTIV was designed to encourage growth in autonomy, but the lack of change in self-efficacy suggested that participants had not internalized behavior change strategies and that exercises and activity were not sustained after the reminders stopped. Brawley et al30 asserted that for adults with chronic disease or disability, even minor illness or injury may reduce activity. Therefore, ongoing support may be required to improve or even maintain physical activity for people living in the community after stroke. At present, the majority of stroke services are unable to offer ongoing follow-up for people after stroke, particularly for those in rural areas.31 We found that ACTIV is a safe intervention that may improve physical activity for some people after stroke, as evidenced by the per-protocol results. Using readily accessible low-cost technology means ACTIV could be offered to people who are discharged to rural areas to extend their poststroke rehabilitation.4 Interventions using simple text messages on devices most people own and use every day could extend the reach of care. However, this extension would be finite, and there is a clear need for tools to extend rehabilitation further, with minimal health care professional contact. The advance in technology and increase in digital content has allowed the development of web-based therapeutic interventions. Many of these interventions have little to no ongoing human involvement in their delivery.32 Brooks et al33 found a reduction in pain and an increase in activity in people with knee osteoarthritis through a self-guided and web-based exercise program. A systematic review has also shown that web-based interventions can significantly increase physical activity.34 The ACTIV research team is investigating how to use the key components of ACTIV in a self-guided, web-based program.
Strengths of This Trial
The ACTIV trial had broad inclusion criteria and identified people at admission to hospital to minimize the chance of missing potential participants. The prevalence of cognitive deficit following even a mild stroke is around 50%.35 ACTIV’s low threshold for cognitive ability allowed inclusion of participants who are often excluded from intervention studies. The physical therapists who delivered the ACTIV program were not specialists in neurological therapy but were able to personalize the program within clear guidelines. Previous research has emphasized the need to include a degree of personalization in any widely disseminated program to reflect the needs and personal preferences of the recipient.36 The sensitivity analysis supports the robustness of our findings (Supplemental material 1, Figure II).
Limitations of This Trial
Many people required rehabilitation but were either unable to be contacted or did not wish to receive ACTIV. This means that only a small number of potential participants received ACTIV, which limited the generalizability of ACTIV to the whole stroke population. Several emerging technologies are being investigated for their utility in delivering telerehabilitation to people with stroke—for example, tablets and smart phones. However, the ACTIV trial focused only on the use of devices already owned by most people. We only used 1-way messaging to reduce complexity and cost; the addition of 2-way communication might have increased engagement. The available funding was insufficient to allow the use of multilingual text messages, which might have broadened the reach of ACTIV. We addressed this issue by encouraging the support of family and friends to translate where needed, but there might have been people who did not have the requisite support to receive ACTIV. The decision to exclude cerebellar and brainstem strokes followed other large intervention trials37 but did reduce generalizability of ACTIV.
Conclusions
The findings of this trial showed that rehabilitation augmented using readily accessible technology did not improve physical activity for those in the intervention group, although there was a significant between-group difference in participation and quality of life. Those who received at least 2 visits, 3 phone calls, and half of the text messages (per-protocol analysis) showed improved physical function following the intervention, but this was not sustained after the program ended. Ongoing input seems to be required to halt the decline patients frequently experience after discharge from rehabilitation. ACTIV may offer a solution to the problem of extending stroke rehabilitation but will not be suitable for all patients discharged from rehabilitation.
Supplemental Material
Supplemental material, Additional_file_1_supplemental_material_1 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair
Supplemental material, Additional_file_2_supplemental_material_2 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair
Supplemental material, CONSORT_Checklist_FINAL_Aug_2020 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair
Acknowledgments
The authors would like to acknowledge the members of the Data Management Committee, Dr Nada Signal, Dr Gwyn Lewis, Steve Taylor, and the physiotherapy staff and patients in the clinical centers.
Footnotes
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at http://nnr.sagepub.com/content/by/supplemental-data.
Authors’ Note: NLS and DT conceived the study and developed the ACTIV program with SM and LH. NLS had oversight of the trial and was responsible for the day-to-day management. ACV advised on study design and was the trial statistician. SM and VF were expert advisors on the study and SM collected data on intervention fidelity. All authors were part of the team that was successful in attracting grant funding. PB contributed to the planning of the trial and advised on data collection. CH and LH assisted with management of recruitment centers. All authors read, commented, and agreed on the final draft of this article. Ethical approval was obtained from The New Zealand Multi-regional Ethics Committee (MEC 11/11/089). All participants provided signed informed consent. Data collected for this trial are available from the corresponding author upon reasonable request. Trial registration, URL: https://www.ctc.usyd.edu.au. Unique identifier: ACTRN12612000464864.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Health Research Council of New Zealand 11/545.
ORCID iDs: Nicola L. Saywell  https://orcid.org/0000-0003-4676-3444
https://orcid.org/0000-0003-4676-3444
Suzie Mudge  https://orcid.org/0000-0003-3106-6777
https://orcid.org/0000-0003-3106-6777
Carl Hanger  https://orcid.org/0000-0001-7952-3936
https://orcid.org/0000-0001-7952-3936
References
- 1. National Stroke Foundation. Clinical guidelines for stroke management 2010. Accessed October 23, 2020 https://www.pedro.org.au/wp-content/uploads/CPG_stroke.pdf
- 2. Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11:459-484. [DOI] [PubMed] [Google Scholar]
- 3. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. 2018;319:820-821. [DOI] [PubMed] [Google Scholar]
- 4. Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314:1255-1263. [DOI] [PubMed] [Google Scholar]
- 5. Buchholz SW, Wilbur J, Ingram D, Fogg L. Physical activity text messaging interventions in adults: a systematic review. Worldviews Evid Based Nurs. 2013;10:163-173. [DOI] [PubMed] [Google Scholar]
- 6. Tieges Z, Mead G, Allerhand M, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil. 2015;96:15-23. [DOI] [PubMed] [Google Scholar]
- 7. Saywell N, Vandal AC, Brown P, et al. Telerehabilitation to improve outcomes for people with stroke: study protocol for a randomised controlled trial. Trials. 2012;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saywell N, Taylor D. Focus group insights assist trial design for stroke telerehabilitation: a qualitative study. Physiother Theory Pract. 2015;31:160-165. [DOI] [PubMed] [Google Scholar]
- 9. Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175-1184. [DOI] [PubMed] [Google Scholar]
- 10. Callahan CMMD, Unverzagt FWP, Hui SLP, Perkins AJMS, Hendrie HCMBC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771-781. [DOI] [PubMed] [Google Scholar]
- 11. Saywell NL. Augmented Community Telerehabilitation Intervention to improve outcomes for people with stroke. ACTIV: a randomised controlled trial and qualitative enquiry 2016. Accessed October 23, 2020 https://openrepository.aut.ac.nz/bitstream/handle/10292/10785/SaywellLS.pdf?sequence=4&isAllowed=y
- 12. Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88:1314-1319. [DOI] [PubMed] [Google Scholar]
- 13. Lin KC, Fu T, Wu CY, et al. Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabil Neural Repair. 2010;24:486-492. [DOI] [PubMed] [Google Scholar]
- 14. Duncan PW, Bode RK, Lai SM, Perera S. Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84:950-963. [DOI] [PubMed] [Google Scholar]
- 15. Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0: evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131-2140. [DOI] [PubMed] [Google Scholar]
- 16. Bohannon RW. Adequacy of hand-grip dynamometry for characterizing upper limb strength after stroke. Isokinet Exerc Sci. 2004;12:263-265. [Google Scholar]
- 17. Hill K, Bernhardt J, McGann A, Maltese D, Berkovits D. A new test of dynamic standing balance for stroke patients: reliability, validity and comparison with healthy elderly. Physiother Can. 1996;48:257-262. [Google Scholar]
- 18. Jones F, Partridge C, Reid F. The Stroke Self-Efficacy Questionnaire: measuring individual confidence in functional performance after stroke. J Clin Nurs. 2008;17:244-252. [DOI] [PubMed] [Google Scholar]
- 19. Duncan PW, Reker D, Kwon S, et al. Measuring stroke impact with the Stroke Impact Scale: telephone versus mail administration in veterans with stroke. Med Care. 2005;43:507-515. [DOI] [PubMed] [Google Scholar]
- 20. Hunger M, Sabariego C, Stollenwerk B, Cieza A, Leidl R. Validity, reliability and responsiveness of the EQ-5D in German stroke patients undergoing rehabilitation. Qual Life Res. 2012;21:1205-1216. [DOI] [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE): version 4. Accessed October 23, 2020 https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- 22. White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marsden D, Quinn R, Pond N, et al. A multidisciplinary group programme in rural settings for community-dwelling chronic stroke survivors and their carers: a pilot randomized controlled trial. Clin Rehabil. 2010;24:328-341. [DOI] [PubMed] [Google Scholar]
- 24. Van De Port IGL, Kwakkel G, Van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37:167-171. [DOI] [PubMed] [Google Scholar]
- 25. Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis. 2018;27:2306-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen J, Jin W, Zhang XX, Xu W, Liu XN, Ren CC. Telerehabilitation approaches for stroke patients: systematic review and meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis. 2015;24:2660-2668. [DOI] [PubMed] [Google Scholar]
- 27. Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. 2013;(12):CD010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flansbjer UB, Downham D, Lexell J. Knee muscle strength, gait performance, and perceived participation after stroke. Arch Phys Med Rehabil. 2006;87:974-980. [DOI] [PubMed] [Google Scholar]
- 29. Wiles R, Ashburn A, Payne S, Murphy C. Discharge from physiotherapy following stroke: the management of disappointment. Soc Sci Med. 2004;59:1263-1273. [DOI] [PubMed] [Google Scholar]
- 30. Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3, suppl 2):172-183. [DOI] [PubMed] [Google Scholar]
- 31. Adams R, Jones A, Lefmann S, Sheppard L. Towards understanding the availability of physiotherapy services in rural Australia. Rural Remote Health. 2016;16:3686. [PubMed] [Google Scholar]
- 32. Barak A, Klein B, Proudfoot JG. Defining internet-supported therapeutic interventions. Ann Behav Med. 2009;38:4-17. [DOI] [PubMed] [Google Scholar]
- 33. Brooks MA, Beaulieu JE, Severson HH, et al. Web-based therapeutic exercise resource center as a treatment for knee osteoarthritis: a prospective cohort pilot study. BMC Musculoskelet Disord. 2014;15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jahangiry L, Farhangi MA, Shab-Bidar S, Rezaei F, Pashaei T. Web-based physical activity interventions: a systematic review and meta-analysis of randomized controlled trials. Public Health. 2017;152:36-46. [DOI] [PubMed] [Google Scholar]
- 35. Jacquin A, Binquet C, Rouaud O, et al. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis. 2014;40:1029-1038. [DOI] [PubMed] [Google Scholar]
- 36. Brady MC, Stott DJ, Norrie J, et al. Developing and evaluating the implementation of a complex intervention: using mixed methods to inform the design of a randomised controlled trial of an oral healthcare intervention after stroke. Trials. 2011;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langhammer B, Stanghelle JK, Lindmark B. An evaluation of two different exercise regimes during the first year following stroke: a randomised controlled trial. Physiother Theory Pract. 2009;25:55-68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Additional_file_1_supplemental_material_1 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair
Supplemental material, Additional_file_2_supplemental_material_2 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair
Supplemental material, CONSORT_Checklist_FINAL_Aug_2020 for Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial by Nicola L. Saywell, Alain C. Vandal, Suzie Mudge, Leigh Hale, Paul Brown, Valery Feigin, Carl Hanger and Denise Taylor in Neurorehabilitation and Neural Repair