Abstract
BACKGROUND
Focal nodal hyperplasia (FNH) is a common benign tumor of the liver. It occurs mostly in people aged 40-50 years and 90% of the patients are female. FNH can be cured by local resection. How to locate and judge the tumor boundary in real time is often a challenge for surgeons.
AIM
To summarize the technique and feasibility of robotic resection of FNH guided by indocyanine green (ICG) fluorescence imaging.
METHODS
The demographics and perioperative outcomes of a consecutive series of patients who underwent robotic resection of liver FNH guided by ICG fluorescence imaging between May 1, 2018 and September 30, 2019 were retrospectively analyzed. ICG was injected through the median elbow vein in all the patients at a dose of 0.25 mg/kg 48 h before the operation. During the operation, the position of FNH in the liver was located in the fluorescence mode of the Da Vinci Si robot operating system and the tumor boundary was determined during the resection.
RESULTS
Among the 23 patients, there were 11 males and 12 females, with a mean age of 30.5 ± 9.3 years. Twenty-two cases completed robotic resection, while one (4.3%) case converted to open surgery. In the robotic surgery group, the operation time was 35-340 min with a median of 120 min, the intraoperative bleeding was 10-800 mL with a median of 50 mL, and the postoperative hospital stay was 1-7 d with a median of 4 d. Biliary fistula occurred in two (8.7%) patients after robotic operation and they both recovered after conservative treatment. One (4.3%) patient received blood transfusion and there was no death in this study. The postoperative hospital stay in the small tumor group was significantly shorter than that in the large tumor group (P < 0.05).
CONCLUSION
ICG fluorescence imaging can guide the surgeon to perform robotic resection of liver FNH by locating the tumor and displaying the tumor boundary in real time. It is a safe and feasible method to ensure the complete resection of the tumor.
Keywords: Robotic surgery, Indocyanine green, Focal nodal hyperplasia, Hepatectomy
Core Tip: Focal nodal hyperplasia (FNH) is a common benign tumor of the liver, which can be cured by local resection. The purpose of this study was to summarize the technique and feasibility of robotic resection of FNH guided by indocyanine green fluorescence imaging.
INTRODUCTION
Focal nodal hyperplasia (FNH) is a common benign tumor of the liver. It occurs mostly in people aged 40-50 years and 90% of the patients are female[1]. FNH is the second benign tumor of the liver after cavernous hemangioma. It usually presents as a limited nodule in the liver, which is usually less than 5 cm. So far, there is no report of malignant transformation of FNH in the literature[2]. In the nodal section, there are characteristic central star shaped scars, which are like spokes from the fibrotic axis to the surrounding area[3,4]. The pathogenesis of FNH is not very clear. It was thought to be related to oral contraceptives, but many cases were found to have no history of taking such medicine. It may be related to the process of liver misconstruction, focal damage of liver tissue, and abnormal development of intrahepatic arteries. Most patients have no obvious self-conscious symptoms, which are often found in imaging examination or diagnosis and treatment of other diseases[5-7].
Indocyanine green (ICG), as a diagnostic drug, is often used to check liver function and circulatory function[8]. After ICG is injected intravenously into the body, it immediately binds with plasma protein and rapidly distributes in the blood vessels of the whole body through the blood circulation. ICG can be efficiently and selectively ingested by hepatocytes, and excreted into bile in a free form from hepatocytes. It enters the intestine through the biliary tract and is excreted out of the body with feces. Because of the fast excretion, about 97% of ICG is excluded from the blood in normal people 20 min after intravenous injection. It does not participate in the chemical reaction in vivo, and does not have enterohepatic circulation. It also does not have lymphatic countercurrent, and does not excrete from other extrahepatic organs such as the kidney[9,10].
In recent years, ICG has been used in laparoscopic liver tumor resection, detection of metastatic lymph nodes in breast cancer, bile duct enhancement, and adrenalectomy[11-16]. The purpose of this study was to summarize the technique and feasibility of robotic resection of FNH guided by ICG fluorescence imaging.
MATERIALS AND METHODS
Patients
The clinical data of 23 consecutive patients who underwent robotic resection of liver FNH guided by ICG fluorescence imaging between May 1, 2018 and September 30, 2019 were retrospectively analyzed.
Patient selection
The inclusion criteria were: (1) Presence of a resectable liver FNH; and (2) No general medical conditions that are contraindicated for anesthesia and surgery. The exclusion criteria were: (1) Tumors larger than 10 cm; and (2) Multiple tumors in the liver.
Preoperative evaluation
Magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) was performed as a routine diagnostic procedure. CT angiography and three-dimensional reconstruction were also performed in all patients for preoperative assessment and surgical planning.
Perioperative data
The baseline demographics and perioperative and pathology data were obtained from the electronic medical records. The clinical outcomes, including operative time, estimated blood loss (EBL), postoperative complications, and postoperative hospital stay (PHS), were analyzed retrospectively. Postoperative biliary fistula was defined as the outflow of bile or bile containing fluid from the abdominal drainage tube.
Surgical technique and follow-up
All the operations in this study were performed by a single team of surgeons using the Da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, United States). This team had performed more than 3000 robotic hepatopancreatobiliary procedures. Forty-eight hours before the operation, ICG was injected through the median elbow vein in all the patients at a dose of 0.25 mg/kg. The patients were placed in the supine decubitus position under general anesthesia. Four or five ports were placed depending on the tumor location. After docking, dissection and mobilization of the liver were performed using a coagulation hook or an ultrasonic scalpel. In the fluorescent mode, we examined the FNH location on the liver surface. For FNH located in liver parenchyma, intraoperative ultrasound was used to locate the tumor and the boundary of FNH was determined in the fluorescence mode after liver parenchyma has been split. Depending on the location of the tumor in the liver, the operation methods included local resection, segmental resection, and hemihepatectomy.
All patients were followed 1 mo after discharge and then at 3-mo intervals thereafter.
Statistical analysis
Continuous data are presented as the mean ± SD or median and interquartile range (IQR) according to their distributions. The Student’s t-test was used to compare normally distributed variables between groups, whereas the Mann–Whitney U test was used for non-normally distributed variables. Categorical data were compared using the Chi-squared test. A P value < 0.05 was considered statistically significant. All analyses were performed with IBM SPSS statistical software, version 20 (SPSS; Chicago, IL, United States).
RESULTS
Patient characteristics
Table 1 shows the detailed characteristics of the 23 patients. The patients included 11 men and 12 women with a mean age of 30.5 years. The most common tumor site was segment IV (6; 26%), followed by segment II/III (4; 17%) and segment V/VI (4; 17%), segment VII (3; 13%) and segment VIII (3; 13%), segment I (2; 9%), and the left half liver (1; 4%). The mean largest tumor diameter was 5.6 cm and 13 patients had a tumor larger than 5 cm. All the tumors were FNH on final histopathological examination.
Table 1.
Patient characteristics
|
Patient No./sex/age (yr)
|
Location segment
|
Operation time (min)
|
EBL (mL)
|
Tumor size (cm)
|
PHS (d)
|
| 1/F/28 | II/III | 80 | 20 | 5 | 4 |
| 2/M/20 | IVb | 50 | 10 | 3.5 | 3 |
| 3/M/21 | IVb | 90 | 50 | 4.5 | 3 |
| 4/F/36 | VII | 130 | 20 | 5 | 6 |
| 5/M/32 | Iva | 123 | 200 | 3 | 1 |
| 6/F/29 | I | 150 | 100 | 4 | 3 |
| 7/M/49 | II/III | 70 | 20 | 4.3 | 1 |
| 8/M/31 | VII | 230 | 400 | 4 | 51 |
| 9/F/14 | V/VI | 280 | 300 | 13 | 72 |
| 10/M/48 | VI | 137 | 200 | 5 | 42 |
| 11/F/40 | IVb | 120 | 10 | 8.5 | 2 |
| 12/M/25 | VIII | 200 | 200 | 5.4 | 4 |
| 13/M/24 | VI | 150 | 200 | 11 | 6 |
| 14/M/37 | VI | 41 | 20 | 2 | 3 |
| 15/F/35 | VII | 90 | 50 | 5.2 | 4 |
| 16/F/36 | II/III | 130 | 50 | 2.2 | 4 |
| 17/M/30 | I | 125 | 100 | 7 | 3 |
| 18/M/28 | IVb | 100 | 50 | 6 | 4 |
| 19/M/29 | VIII | 100 | 100 | 5 | 4 |
| 20/F/31 | VIII | 60 | 10 | 3.5 | 2 |
| 21/F/24 | II/III | 35 | 10 | 9 | 4 |
| 22/F/13 | II/III/IV | 340 | 800 | 10 | 63 |
| 23/F/41 | IVb | 60 | 10 | 3 | 3 |
Indicates conversion to open surgery.
Indicates postoperative biliary fistula.
Indicates intraoperative blood transfusion. EBL: Estimated blood loss; PHS: Postoperative hospital stay; F: Female; M: Male.
Perioperative outcomes
The FNH of 22 patients was successfully removed robotically and one patient converted to open surgery. The mean operative time of robotic resection was 121 min, and the median EBL of robotic resection was 115 mL (IQR: 10-800 mL). One patient required blood transfusion. Postoperative morbidities occurred in two (8.7%) patients, who had postoperative biliary fistula. The mean PHS was 3.7 d. No patients required readmission to hospital and there was no 90-d mortality. After a median follow-up of 12 mo (IQR: 4-17 mo), no patient showed radiologic evidence of tumor recurrence.
Surgical findings
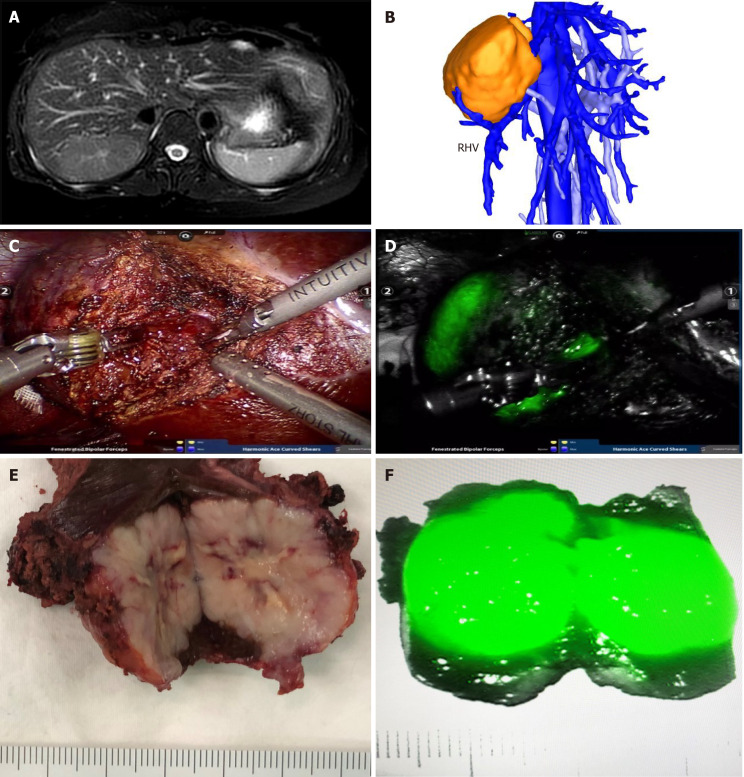
In all patients, FNH in the liver showed bright green imaging in the robot fluorescence mode. All tumors were evenly imaged, and the rest normal liver tissues were not imaged. For FNH protruding from the surface of the liver, it was very easy to locate the tumor during the operation. For FNH located in the liver parenchyma, combined with intraoperative ultrasound, all tumors were successfully found and completely removed. In the liver parenchyma, ICG imaging accurately displayed the tumor boundary in real time, and the operation was safe and feasible. Preoperative MRI of patient number 14 showed that the tumor was located in segment VII. Three dimensional reconstruction of CT images showed that the tumor crossed the right hepatic vein. During the operation, ICG real-time imaging accurately showed the location of the tumor and ensured the scope of operation. The postoperative specimens showed that the tumors were uniformly stained by ICG, and the imaging effect was satisfactory (Figure 1).
Figure 1.
Robotic resection of liver focal nodal hyperplasia guided by indocyanine green fluorescence imaging. A: Magnetic resonance imaging showed focal nodal hyperplasia (FNH) located in segment VII; B: Three-dimensional reconstruction of computed tomography showed the relationship of FNH and the right hepatic vein; C and D: Robotic resection of liver FNH guided by indocyanine green (ICG) fluorescence imaging; E and F: The resected specimen and the mode of ICG imaging in FNH. RHV: Right hepatic vein.
Clinical outcomes according to tumor size
The 22 patients who underwent robotic resection were divided into two groups according to the tumor size. Table 2 shows the demographic data and perioperative outcomes of these patients. There were no significant differences between the large (≥ 5 cm) and small (< 5 cm) tumor groups of patients with respect to age, sex, and tumor location. The two groups had similar operation times, EBL, and morbidity rates (P > 0.05). The PHS in the small tumor group was significantly shorter than that in the large tumor group (P < 0.05).
Table 2.
Comparison between patients with large (≥ 5 cm) and small (< 5 cm) tumor robotic resection (n = 22)
|
Variable
|
Large tumor (n = 13)
|
Small tumor (n = 9)
|
P
value
|
| Age (yr) | 28.8 ± 9.65 | 32.7 ± 8.71 | 0.831 |
| Percentage of females (%) | 61.5 | 40.0 | 0.414 |
| Operation time (IQR, min) | 80.0 | 77.5 | 0.148 |
| EBL, median (IQR, mL) | 180.0 | 115.0 | 0.284 |
| PHS (IQR, d) | 2.0 | 1.5 | 0.006 |
| Morbidity rate (%) | 15.4 | 10.0 | 1.000 |
IQR: Interquartile range; EBL: Estimated blood loss; PHS: Postoperative hospital stay.
DISCUSSION
FNH is a benign tumor of the liver, which is common in young women. Most cases are asymptomatic and the tumor does not undergo malignant transformation, but the tumor volume will increase year by year in some patients, and surgical resection is often effective[17]. FNH is considered to be a result of the reactive proliferation of hepatocytes to local vascular abnormalities in the liver. The main components of the lesion include hyperplastic hepatocytes, Kupffer cells, bile ducts, and variant arterial vessels. There is no normal arrangement of lobular structure in the tumor. In the gross and pathological sections, there is scar like fibrous tissue in the center of the tumor, which is distributed radially like spoke wheel. FNH is mainly fed by the small branches of the hepatic artery located in the central scar. There is no capillary network in the tumor, and the direction of blood flow is centrifugal.
The clear diagnosis based on FNH imaging is of great significance. The typical scar structure often occurs in the lesions larger than 3 cm, while the lesions smaller than 3 cm often have no scar[18,19]. In MRI examination, FNH usually shows equal or slightly low signal on T1WI, and equal or slightly high signal on T2WI (high signal of central spoke scar on T2WI). Contrast agent uptake is obviously uniform and enhanced in the arterial phase, there is equal or slightly high signal between lesions in the portal phase and delayed phase and liver parenchyma, but there is no (false) capsule and no reduction of signal in reverse phase images[20,21]. Because the rate of star shaped scar appearing on ultrasound is very low, and it can also appear in other liver lesions, whether it appears or not cannot be used as a specific sign of ultrasound diagnosis of FNH. Ultrasound imaging of FNH shows that the central nutrient artery is radiated to the star shape around, and the blood flow velocity of the artery is high and the resistance is low. Contrast-enhanced ultrasound is an effective method for the diagnosis of FNH, with a sensitivity of 83% and specificity of 98%. Its characteristic is that the contrast medium is centrifugally filled from the center to the periphery of the tumor in the form of spokes[22-24].
There is no normal lobular structure in FNH, which is mainly fed by hyperplastic arterioles. There is hyperplastic bile duct in the central scar and no portal vein. When ICG is given intravenously to patients, the hepatocytes in FNH are easy to absorb ICG but difficult to excrete it, while the surrounding normal hepatocytes can metabolize ICG normally, so FNH is easy to be stained by ICG and used for intraoperative detection. In our study, FNH in the liver of all patients was successfully stained by ICG and the imaging effect was good. Due to the lack of a real tumor envelope in FNH, it is difficult to distinguish the tumor boundary from the normal liver tissue in the liver parenchyma, which is also a dilemma that surgeons often face when they are operating to remove FNH[25]. We used the fluorescence mode of the da Vinci robotic system to locate the boundary of FNH in the liver by ICG imaging, which guided and modified the boundary in real time during resection, so as to ensure the smooth and complete resection of FNH in all patients and avoided tumor residual. It was easy to find FNH located on the surface of the liver, while it was often difficult to locate FNH in the liver parenchyma. If the FNH was located in liver parenchyma, as long as a small part of the tumor can be found after the liver parenchyma had been split, ICG imaging could guide the whole surgical resection process.
Local surgical resection can not only achieve the effect of radical cure for FNH, but also keep the normal liver tissue as much as possible, and reduce bleeding and other complications caused by massive hepatectomy. Our study showed that it was safe and effective to demonstrate FNH by ICG imaging and to use da Vinci robot for local resection of FNH. Especially for those patients who cannot undergo regular hepatectomy in special position, ICG imaging can effectively ensure the scope of surgical resection and the complete resection of tumor. In practice, we realized that real-time detection of FNH located in liver parenchyma by ICG imaging is effective for the guidance and correction of the resection boundary of tumor intraoperatively.
ICG has the risk of causing shock and anaphylactic symptoms, so it is necessary to closely observe and detect the vital signs of patients from the beginning of injection to the end of operation, and make preparations for the disposal of emergency drugs and equipment that should be prepared for patients with anaphylaxis. In our study, there were no patients with anaphylaxis and shock symptoms, but some patients developed nausea, fever, and other reactions after administration, which were relieved after symptomatic support treatment. In the instructions for drug use, it is clearly stated that those who have a history of allergy to iodine should not be used, and those who have an allergic constitution should be used with caution. Therefore, before giving ICG to patients, it is necessary to ask them if they have any history of allergy and allergic drugs.
CONCLUSION
In conclusion, our study shows that ICG is an excellent intraoperative dye developer for FNH, and it is safe and effective to use Da Vinci robot to give such patients complete resection of tumor. In the future, more large-scale case accumulation is needed to further summarize the safety and effectiveness of this method.
ARTICLE HIGHLIGHTS
Research background
Focal nodal hyperplasia (FNH) is a common benign tumor of the liver. It occurs mostly in people aged 40-50 years and 90% of the patients are female.
Research motivation
How to locate and judge the tumor boundary in real time is often a challenge for surgeons.
Research objectives
To summarize the technique and feasibility of robotic resection of FNH guided by indocyanine green (ICG) fluorescence imaging.
Research methods
The demographics and perioperative outcomes of a series of consecutive patients who underwent robotic resection of liver FNH guided by ICG fluorescence imaging between May 1, 2018 and September 30, 2019 were retrospectively analyzed. ICG was injected through the median elbow vein in all the patients at a dose of 0.25 mg/kg 48 h before the operation.
Research results
Among the 23 patients, there were 11 males and 12 females, with a mean age of 30.5 ± 9.3 years. Twenty-two cases completed robotic resection, while one (4.3%) case converted to open surgery. In the robotic surgery group, the operation time was 35-340 min with a median of 120 min, the intraoperative bleeding was 10-800 mL with a median of 50 mL, and the postoperative hospital stay was 1-7 d with a median of 4 d. Biliary fistula occurred in two (8.7%) patients after robotic operation and they both recovered after conservative treatment. One (4.3%) patient received blood transfusion and there was no death in this study. The postoperative hospital stay in the small tumor group was significantly shorter than that in the large tumor group (P < 0.05).
Research conclusions
Our study shows that ICG is an excellent intraoperative dye developer for FNH, and it is safe and effective to use Da Vinci robot to give such patients complete resection of tumor. In the future, more large-scale case accumulation is needed to further summarize the safety and effectiveness of this method.
Research perspectives
Cancer treatments are becoming more diverse.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Chinese PLA General Hospital.
Informed consent statement: All patients involved in the present study provided informed written consent.
Conflict-of-interest statement: The authors declare that they have no conflict of interest to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: August 17, 2020
First decision: September 21, 2020
Article in press: October 26, 2020
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Facciorusso A, Seo D, Tanimine N S-Editor: Chen XF L-Editor: Wang TQ P-Editor: Li JH
Contributor Information
Cheng-Gang Li, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China. lcgang301@126.com.
Zhi-Peng Zhou, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Xiang-Long Tan, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Zi-Zheng Wang, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Qu Liu, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Zhi-Ming Zhao, Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Data sharing statement
No additional data are available.
References
- 1.Gürses C, Okşar FS, Erol B, Yalçın M, Kahvecioğlu N, Alparslan AŞ. Natural course of hepatic focal nodular hyperplasia from childhood to adulthood and review of the literature. Turk J Gastroenterol. 2017;28:492–497. doi: 10.5152/tjg.2017.17227. [DOI] [PubMed] [Google Scholar]
- 2.Bröker MEE, Klompenhouwer AJ, Gaspersz MP, Alleleyn AME, Dwarkasing RS, Pieters IC, de Man RA, IJzermans JNM. Growth of Focal Nodular Hyperplasia is Not a Reason for Surgical Intervention, but Patients Should be Referred to a Tertiary Referral Centre. World J Surg. 2018;42:1506–1513. doi: 10.1007/s00268-017-4335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsukuma KE, Yeh MM. Update on the pathology of liver neoplasms. Ann Diagn Pathol. 2019;38:126–137. doi: 10.1016/j.anndiagpath.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Giambelluca D, Taibbi A, Midiri M, Bartolotta TV. The "spoke wheel" sign in hepatic focal nodular hyperplasia. Abdom Radiol . 44:1183–1184. doi: 10.1007/s00261-018-1852-1. [DOI] [PubMed] [Google Scholar]
- 5.van Rosmalen BV, de Graeff JJ, van der Poel MJ, de Man IE, Besselink M, Abu Hilal M, Busch OR, Verheij J, van Gulik TM Dutch Benign Liver Tumour Group. Impact of open and minimally invasive resection of symptomatic solid benign liver tumours on symptoms and quality of life: a systematic review. HPB . 21:1119–1130. doi: 10.1016/j.hpb.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Kitao A, Matsui O, Yoneda N, Kita R, Kozaka K, Kobayashi S, Gabata T. Differentiation Between Hepatocellular Carcinoma Showing Hyperintensity on the Hepatobiliary Phase of Gadoxetic Acid-Enhanced MRI and Focal Nodular Hyperplasia by CT and MRI. AJR Am J Roentgenol. 2018;211:347–357. doi: 10.2214/AJR.17.19341. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Tana C, Caraiani C, Dong Y. Contrast enhanced ultrasound (CEUS) imaging of solid benign focal liver lesions. Expert Rev Gastroenterol Hepatol. 2018;12:479–489. doi: 10.1080/17474124.2018.1464389. [DOI] [PubMed] [Google Scholar]
- 8.Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg Innov. 2016;23:166–175. doi: 10.1177/1553350615604053. [DOI] [PubMed] [Google Scholar]
- 9.Trout AT, Towbin AJ, Smith EA, Gupta A, Dillman JR. Hepatocyte-specific contrast media: not so simple. Pediatr Radiol. 2018;48:1245–1255. doi: 10.1007/s00247-018-4108-x. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M, Namimoto T, Shimizu K, Morita K, Sakamoto F, Oda S, Nakaura T, Utsunomiya D, Shiraishi S, Yamashita Y. Measuring hepatic functional reserve using T1 mapping of Gd-EOB-DTPA enhanced 3T MR imaging: A preliminary study comparing with 99m Tc GSA scintigraphy and signal intensity based parameters. Eur J Radiol. 2017;92:116–123. doi: 10.1016/j.ejrad.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Spinoglio G, Bertani E, Borin S, Piccioli A, Petz W. Green indocyanine fluorescence in robotic abdominal surgery. Updates Surg. 2018;70:375–379. doi: 10.1007/s13304-018-0585-6. [DOI] [PubMed] [Google Scholar]
- 12.Ei S, Itano O, Yoshida H, Ojima H, Shimoda M, Uchida H, Maeda S, Kumamoto Y, Aiko S. The potentiality of laparoscopic partial liver excisional biopsy using analysis of the liver surface based on preoperative 3D simulation imaging: A case report. Int J Surg Case Rep. 2018;45:33–37. doi: 10.1016/j.ijscr.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgilio E, Cavallini M. Managing Focal Nodular Hyperplasia of the Liver: Surgery or Minimally-invasive Approaches? Anticancer Res. 2018;38:33–36. doi: 10.21873/anticanres.12188. [DOI] [PubMed] [Google Scholar]
- 14.Hau HM, Kloss A, Wiltberger G, Jahn N, Krenzien F, Benzing C, Schmelzle M, Seehofer D, Atanasov G, Bartels M. The challenge of liver resection in benign solid liver tumors in modern times - in which cases should surgery be done? Z Gastroenterol. 2017;55:639–652. doi: 10.1055/s-0043-110145. [DOI] [PubMed] [Google Scholar]
- 15.Souzaki R, Kawakubo N, Matsuura T, Yoshimaru K, Koga Y, Takemoto J, Shibui Y, Kohashi K, Hayashida M, Oda Y, Ohga S, Taguchi T. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr Surg Int. 2019;35:551–557. doi: 10.1007/s00383-019-04458-5. [DOI] [PubMed] [Google Scholar]
- 16.Yuan L, Qi X, Zhang Y, Yang X, Zhang F, Fan L, Chen L, Zhang K, Zhong L, Li Y, Gan S, Fu W, Jiang J. Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective single-center randomized study. Cancer Biol Med. 2018;15:452–460. doi: 10.20892/j.issn.2095-3941.2018.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrakis A, Vassos N, Grützmann R, Croner RS. What is Changing in Indications and Treatment of Focal Nodular Hyperplasia of the Liver. Is There Any Place for Surgery? Ann Hepatol. 2017;16:333–341. doi: 10.5604/16652681.1235475. [DOI] [PubMed] [Google Scholar]
- 18.Cannella R, Borhani AA, Minervini MI, Tsung A, Furlan A. Evaluation of texture analysis for the differential diagnosis of focal nodular hyperplasia from hepatocellular adenoma on contrast-enhanced CT images. Abdom Radiol . 44:1323–1330. doi: 10.1007/s00261-018-1788-5. [DOI] [PubMed] [Google Scholar]
- 19.Mamone G, Caruso S, Cortis K, Miraglia R. Complete spontaneous regression of giant focal nodular hyperplasia of the liver: Magnetic resonance imaging evaluation with hepatobiliary contrast media. World J Gastroenterol. 2016;22:10461–10464. doi: 10.3748/wjg.v22.i47.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SS, Kim SH, Song KD, Choi SY, Heo NH. Value of gadoxetic acid-enhanced MRI and diffusion-weighted imaging in the differentiation of hypervascular hyperplastic nodule from small (<3 cm) hypervascular hepatocellular carcinoma in patients with alcoholic liver cirrhosis: A retrospective case-control study. J Magn Reson Imaging. 2020;51:70–80. doi: 10.1002/jmri.26768. [DOI] [PubMed] [Google Scholar]
- 21.Lebert P, Adens-Fauquembergue M, Azahaf M, Gnemmi V, Behal H, Luciani A, Ernst O. MRI for characterization of benign hepatocellular tumors on hepatobiliary phase: the added value of in-phase imaging and lesion-to-liver visual signal intensity ratio. Eur Radiol. 2019;29:5742–5751. doi: 10.1007/s00330-019-06210-y. [DOI] [PubMed] [Google Scholar]
- 22.Bertin C, Egels S, Wagner M, Huynh-Charlier I, Vilgrain V, Lucidarme O. Contrast-enhanced ultrasound of focal nodular hyperplasia: a matter of size. Eur Radiol. 2014;24:2561–2571. doi: 10.1007/s00330-014-3280-0. [DOI] [PubMed] [Google Scholar]
- 23.Katrich AN, Porkhanov VA. [Contrast-enhanced ultrasound in differential diagnosis of focal liver lesions] Khirurgiia . (6):49–59. doi: 10.17116/hirurgia201906149. [DOI] [PubMed] [Google Scholar]
- 24.Verhagen MV, Ciocarlie O, Humphries P, Watson T. Contrast-enhanced ultrasound for multiple liver lesions after bone marrow transplant in a child with leukaemia: Multifocal focal nodular hyperplasia. Ultrasound. 2019;27:122–126. doi: 10.1177/1742271X18795328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang C, Zhang P, Qi X. Digital and intelligent liver surgery in the new era: Prospects and dilemmas. EBioMedicine. 2019;41:693–701. doi: 10.1016/j.ebiom.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.