Abstract
Background:
The benefit of adjuvant chemotherapy (ACT) remains unknown for patients with stage I lung adenocarcinoma (ADC) with spread through air spaces (STAS). This study investigated the effect of adjuvant chemotherapy in stage I ADC/STAS-positive patients.
Methods:
A total of 3346 patients with stage I ADC from five institutions in China were identified from 2009 to 2013, of whom 1082 were diagnosed with STAS (32.3%). By using the Kaplan–Meier method and Cox proportional hazard regression model, we explored the impact of STAS on prognosis, and determined if the use of adjuvant chemotherapy was associated with improved outcomes in patients with stage I ADC/STAS-positive. A validation cohort was also included in this study.
Results:
Patients with stage I ADC/STAS-positive in the primary cohort had unfavorable overall survival (OS) and disease-free survival (DFS). A multivariate Cox regression model confirmed the survival disadvantages of STAS in patients with stage I ADC [OS: hazards ratio (HR) = 1.877, 95% confidence interval (CI): 1.579–2.231; p < 0.001; DFS: HR = 1.895, 95% CI: 1.614–2.225; p < 0.001]. Lobectomy was associated with better OS and DFS than sublobar resection (SR) in both stage IA and IB ADC/STAS-positive. Similar results were observed in the validation cohort. For patients with stage IB ADC/STAS-positive, ACT was revealed as an independent factor for favorable survival (OS: HR = 0.604, 95% CI: 0.397–0.919; p = 0.018; DFS: HR = 0.565, 95% CI: 0.372–0.858; p = 0.007). However, among patients with stage IA ADC/STAS-positive, ACT was associated with improved outcomes only for those undergoing SR (OS: HR = 0.787, 95% CI: 0.359–0.949; p = 0.034; DFS: HR = 0.703, 95% CI: 0.330–0.904; p = 0.029).
Conclusion:
The presence of STAS was correlated with poor prognosis in patients with stage I ADC. Our study suggested that ACT might be considered for patients with stage IB ADC/STAS-positive and those with stage IA ADC/STAS-positive who underwent SR.
Keywords: adjuvant chemotherapy, lung adenocarcinoma, spread through air spaces, surgical procedures, survival
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide.1 Nowadays, surgical resection remains the primary treatment for early-stage non-small cell lung cancer (NSCLC). Although adjuvant chemotherapy (ACT) has been recommended for patients with resected stage II and IIIA NSCLC,2 its role in patients with stage I NSCLC is still inconclusive. Current National Comprehensive Cancer Network guidelines suggest that ACT should be considered in stage IB patients with high-risk factors, including tumor size >4 cm, poorly differentiated lymphovascular invasion, visceral pleura invasion, incomplete lymph node sampling, or wedge resection.3 However, whether stage IB patients obtain any survival benefits from ACT is unclear, owing to a lack of robust evidence. In addition, the definition of stage IB disease has been revised in the current 8th TNM staging system,4 which highlights the necessity to assess survival benefits from ACT in patients with stage IB NSCLC.
Adenocarcinoma (ADC) is the commonest histologic type of NSCLCs, accounting for approximately 50%.5 According to the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) classification of 2011, invasive ADC is categorized into several subtypes based on the predominant pattern, including lepidic, acinar, papillary, solid, and micropapillary, as well as invasive mucinous adenocarcinoma.6 Hitherto, several studies have reported potential survival advantages of ACT in stage IB patients with high-grade (micropapillary/solid-predominant) ADC.7,8 In recent years, tumor spread through air spaces (STAS), has been recognized as a novel invasion pattern in stage I ADCs, which was associated with poor recurrence-free and overall survival.9–11 Increasing evidence has revealed a potential relationship between resection types and patient survival in STAS-positive ADC.10,12,13 More specifically, lobectomy might offer better outcomes than sublobar resection (SR) in STAS-positive T1 ADC.13,14 Besides, our previous study observed that the unfavorable prognosis of patients with stage IA ADC > 2 to 3 cm/STAS-positive was similar to that of patients with stage IB ADC.14 Nonetheless, there has been no consensus on the use of ACT in stage IA patients with STAS-positive ADC, especially in patients receiving different surgical procedures.
In the present study, we performed a survival analysis using a large multi-institutional cohort to investigate the benefits of ACT for stage IB patients with STAS-positive ADC who underwent different types of resection. We also explored the potential benefits of ACT in patients with stage IA ADC/STAS-positive who underwent SR.
Materials and methods
Patient selection
The Institutional Review Boards of the five hospitals approved this study (IRB NO.JD-HG-2020-09). In cases in which individual patient consent was not identified, the chairpersons of the ethics committees from the participating institutions waived the need for patient consent. Patients with pT1-2aN0M0 invasive ADC (stage IA and IB) who underwent R0 pulmonary resection at five medical centers in China from January 2009 to December 2013 were reviewed. To exclude distant metastasis, all patients underwent a preoperative routine examination, including enhanced chest computed tomography (CT) scan, enhanced abdominal CT scan or ultrasonography, fiberoptic bronchoscopy, cranial magnetic resonance imaging, and bone scan. A positron emission tomography (PET)–CT scan was suggested if possible. Patients were excluded from the study cohort if one of the following criteria was met: (1) receipt of induction therapy; (2) presence of multiple primary lung cancers; (3) receipt of targeted therapy or immunotherapy; (4) lack of information on lymph nodes status. Platinum-based ACT (cisplatin plus docetaxel or pemetrexed) was performed in patients with high-risk factors including poor differentiated tumor, visceral pleural invasion, vascular invasion, and SR.8 Age, performance status, and patients’ preference were also considered. No mortality related to chemotherapy was detected among patients who underwent four cycles of ACT. Moreover, we also included a validation cohort (328 patients with stage IA ADC and 251 patients with stage IB ADC) from the Second Affiliated Hospital of Soochow University, with the same criteria, between January 2014 and December 2016.
Histopathologic evaluation of STAS
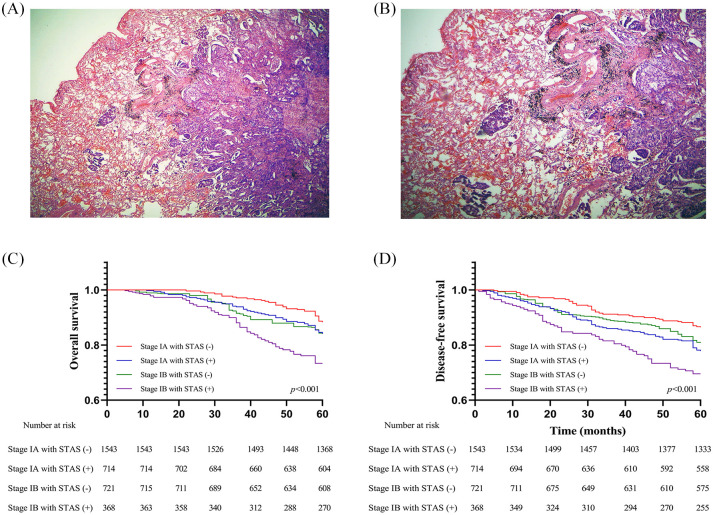
All specimens were formalin-fixed immediately after resection and stained with hematoxylin and eosin. The slides were reviewed independently by pathologists from respective institutions who were blinded to the patient data. Histological classification was evaluated according to the IASLC/ATS/ERS classification of ADC.6 Tumors were classified into lepidic, acinar, papillary, micropapillary, or solid-predominant types when a pattern was predominant in the tumor (even if <50%). The morphological definition of STAS was consistent with that of Travis and Kadota et al.,15,16 and identification of STAS was described in our previous studies.17,18 In brief, lesions of STAS consist of tumor cells that appear morphologically to be situated within normal air spaces as micropapillary clusters, solid nests, or scattered discohesive single cells. In order to avoid confusion with artificially detached cells during tumor dissection, at least three tumor slices were observed under the microscope. In addition, distance between the tumor surface and farthest STAS from the tumor edge was measured using a ruler. Because lung specimens were not consistently inflated during processing, to account for artifactual atelectasis, we also measured according to the number of alveolar spaces. Low- and high-power STAS views are shown in Figure 1A,B.
Figure 1.
(A) Low-power view and (B) high-power view of tumor spread through air spaces in lung ADC (original magnification: ×40 in A and ×100 in B); (C) OS, and (D) DFS of patients with stage I ADC stratified by STAS in the primary cohort.
ADC, adenocarcinoma; DFS, disease-free survival; OS, overall survival; STAS, spread through air spaces.
Postoperative follow up
Patients were followed up every 3 months for the first year after surgery and at 6-month intervals thereafter. For patients who were followed up at local health facilities, survival status and examination results were collected by telephone or email. Tumor locoregional recurrence or distant metastasis was diagnosed using chest CT, brain magnetic resonance imaging (MRI), and bone scintigraphy as well as ultrasound and/or abdominal CT. A PET–CT scan was suggested if possible. Disease-free survival (DFS) was defined as the length of time from surgery to tumor recurrence or the last follow up. Overall survival (OS) was defined as the time from the surgical resection until death from any cause or the last follow up.
Statistical analysis
Associations between clinicopathological characteristics were analyzed using the Pearson χ2 test or Fisher’s exact test for categorical variables. DFS and OS were evaluated using the Kaplan–Meier method, and nonparametric group comparisons were performed using the log-rank test. A Cox proportional-hazards regression model was applied to assess the independent risk factors for DFS and OS. The variables were examined firstly using univariate analyses, and those with p values < 0.1 were incorporated into a multivariate model. Cumulative incidence analysis was used to estimate the cumulative incidence of recurrence (CIR) of locoregional and distant recurrence. All p values were based on two-tailed statistical analyses, and p < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (Statistical Program for Social Sciences 25.0; IBM Corporation, Armonk, NY, USA).
Results
Patient clinicopathological characteristics
We identified 3346 and 579 patients with stage I ADC in the primary and validation cohort, respectively. The median number of alveolar spaces between the tumor and the furthest STAS was six in the primary cohort and seven in the validation cohort. In addition, the median distance between tumor surface and the furthest STAS was measured microscopically as 1.3 mm (range, 0.4–6.8 mm) in the primary cohort and 1.1 mm (range, 0.3–7.0 mm).
The detailed clinicopathological characteristics of all patients stratified by STAS and ACT are summarized in Table 1 and Supplemental Table S1. Patients with stage IA ADC accounted for more than 2/3 in the primary cohort, while patients with stage IA ADC took up more than half in the validation cohort. The presence of STAS was found in 1082 ADCs (32.3%) in the primary cohort and 162 ADCs (28.0%) in the validation cohort. Notably, STAS was more likely to be observed in patients with micropapillary-predominant and solid-predominant ADCs in both cohorts. Additionally, the majority of STAS-positive patients underwent lobectomy in both the primary (59.1%) and the validation (64.8%) cohorts. Meanwhile, only a minority of STAS-positive patients received ACT in both cohorts.
Table 1.
Clinicopathological characteristics of 3346 patients with stage I lung ADC in the primary cohort.
| Variables | STAS |
Adjuvant chemotherapy |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Positive (%) | Negative (%) | p | No. of patients | With (%) | Without (%) | p | |
| Overall | 3346 | 1082 (32.3) | 2264 (67.7) | <0.001 | 3346 | 509 (15.2) | 2837 (84.8) | <0.001 |
| Age (years) | 0.282 | 0.283 | ||||||
| ⩽65 | 2325 | 657 (28.3) | 1668 (71.7) | 2325 | 299 (12.9) | 2026 (87.1) | ||
| >65 | 1021 | 425 (41.6) | 596 (58.4) | 1021 | 210 (20.6) | 811 (79.4) | ||
| Sex | 0.595 | 0.881 | ||||||
| Male | 1854 | 629 (33.9) | 1225 (66.1) | 1854 | 269 (14.5) | 1585 (85.5) | ||
| Female | 1492 | 453 (30.4) | 1039 (69.6) | 1492 | 240 (16.1) | 1252 (83.9) | ||
| Smoking | 0.671 | 0.447 | ||||||
| Nonsmoker | 1877 | 542 (28.9) | 1335 (71.1) | 1877 | 291 (15.5) | 1586 (84.5) | ||
| Current or former smoker | 1469 | 540 (36.8) | 929 (63.2) | 1469 | 218 (14.8) | 1251 (85.2) | ||
| T stage | <0.001 | <0.001 | ||||||
| T1a | 768 | 196 (25.5) | 572 (74.5) | 768 | 16 (2.1) | 752 (97.9) | ||
| T1b | 669 | 236 (35.3) | 433 (64.7) | 669 | 29 (4.3) | 640 (95.7) | ||
| T1c | 820 | 282 (34.4) | 538 (65.6) | 820 | 47 (5.7) | 773 (94.3) | ||
| T2a | 1089 | 368 (33.8) | 721 (66.2) | 1089 | 417 (38.3) | 672 (61.7) | ||
| Histologic pattern | <0.001 | <0.001 | ||||||
| Lepidic predominant | 932 | 170 (18.2) | 762 (81.8) | 932 | 83 (8.9) | 849 (91.1) | ||
| Acinar predominant | 1003 | 320 (31.9) | 683 (68.1) | 1003 | 125 (12.5) | 878 (87.5) | ||
| Papillary predominant | 600 | 198 (33.0) | 402 (67.0) | 600 | 91 (15.2) | 509 (84.8) | ||
| Micropapillary predominant | 256 | 127 (49.6) | 129 (50.4) | 256 | 65 (25.4) | 291 (74.6) | ||
| Solid predominant | 555 | 267 (48.1) | 288 (51.9) | 555 | 145 (26.1) | 410 (73.9) | ||
| Type of surgical resection | 0.736 | 0.869 | ||||||
| Thoracotomy | 277 | 132 (47.7) | 145 (52.3) | 277 | 108 (39.0) | 169 (61.0) | ||
| VATS | 3069 | 950 (31.0) | 2119 (69.0) | 3069 | 401 (13.1) | 2668 (86.9) | ||
| Surgical procedure | <0.001 | <0.001 | ||||||
| Lobectomy | 1514 | 640 (42.3) | 874 (57.7) | 1514 | 302 (19.9) | 1212 (80.1) | ||
| Sublobar resection | 1832 | 442 (24.1) | 1390 (75.9) | 1832 | 207 (11.3) | 1625 (88.7) | ||
| Presence of STAS | 1082 | 198 (18.3) | 884 (81.7) | <0.001 | ||||
ADC, adenocarcinoma; STAS, spread through air spaces; VATS, video-assisted surgery.
The median follow-up time for patients in the primary cohort was 90.8 months, ranging from 80.3 to 139.8 months; while that for patients in the validation cohort was 56.5 months, ranging from 43 to 78 months. The median OS was 65 months (range, 5.4 months to 131.5 months) in the primary cohort and 60 months (range, 8.2 months to 72.7 months) in the validation cohort. Meanwhile, the median DFS was 31 months (range, 1.6 months to 73.5 months) in the primary cohort and 35 months (6.8 months to 63.8 months) in the validation cohort.
STAS affects patient survival in stage I ADC
As shown in Figure 1C–D, survival analysis indicated that patients with STAS suffered from inferior OS (p < 0.001) and DFS (p < 0.001) compared with those without STAS in the primary cohort. A similar result was also seen in the validation cohort (Supplemental Figure S1A,B). Notably, it was observed that patients with stage IA ADC/STAS-positive had similar OS (primary cohort: p = 0.992; validation cohort: p = 0.837) and DFS (primary cohort: p = 0.202; validation cohort: p = 0.923) to patients with stage IB ADC/STAS-negative in both cohorts (Figure 1 and Supplemental Figure S1). Multivariate Cox regression modeling confirmed the survival disadvantages of STAS in patients with stage I ADC [primary cohort: OS: hazard ratio (HR) = 1.877, 95% confidence interval (CI): 1.579–2.231; p < 0.001; DFS: HR = 1.895, 95% CI: 1.614–2.225; p < 0.001; Validation cohort: OS: HR = 2.776, 95% CI: 1.658–4.650; p < 0.001; DFS: HR = 2.854, 95% CI: 1.727–4.716; p < 0.001] in both cohorts (Supplemental Tables S2, S3).
Table 2.
Cox proportional-hazards regression model for OS and DFS in patients with stage IA lung ADC in the primary cohort.
| Variables | STAS-negative |
STAS-positive |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS | OS | DFS | |||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| p | HR (95% CI) | p | p | HR (95% CI) | p | p | HR (95% CI) | p | p | HR (95% CI) | p | |
| T stage | 0.036 | 0.043 | 0.090 | 0.087 | 0.008 | <0.001 | 0.060 | 0.003 | ||||
| T1a | 0.658 (0.471–0.920) | 0.015 | 0.717 (0.527–0.976) | 0.035 | 0.457 (0.285–0.734) | 0.001 | 0.516 (0.339–0.784) | 0.002 | ||||
| T1b | 0.768 (0.546–1.079) | 0.129 | 0.785 (0.568–1.083) | 0.140 | 0.429 (0.273–0.674) | <0.001 | 0.614 (0.422–0.894) | 0.011 | ||||
| T1c | 1 | 1 | 1 | 1 | ||||||||
| Histologic Pattern | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Lepidic predominant | 0.636 (0.413–0.979) | 0.040 | 0.719 (0.471–1.097) | 0.126 | 0.256 (0.138–0.472) | <0.001 | 0.361 (0.216–0.604) | <0.001 | ||||
| Acinar predominant | 0.270 (0.148–0.492) | <0.001 | 0.517 (0.317–0.844) | 0.008 | 0.491 (0.278–0.869) | 0.015 | 0.545 (0.331–0.899) | 0.017 | ||||
| Papillary predominant | 0.647 (0.393–1.066) | 0.087 | 0.913 (0.572–1.455) | 0.701 | 0.582 (0.273–1.244) | 0.163 | 0.645 (0.337–1.235) | 0.186 | ||||
| Micropapillary predominant | 1.706 (1.180–2.469) | 0.005 | 1.986 (1.368–2.882) | <0.001 | 1.007 (0.580–1.747) | 0.982 | 1.006 (0.615–1.646) | 0.981 | ||||
| Solid predominant | 1 | 1 | 1 | 1 | ||||||||
| Surgical Procedure (Lob versus Sublobar) | <0.001 | 0.838 (0.517–1.359) | 0.475 | 0.033 | 1.103 (0.741–1.642) | 0.629 | <0.001 | 0.509 (0.353–0.733) | <0.001 | <0.001 | 0.470 (0.342–0.647) | <0.001 |
| Adjuvant Chemotherapy (with versus without) | <0.001 | 1.758 (0.992–3.116) | 0.053 | 0.042 | 1.356 (0.734–2.508) | 0.331 | 0.453 | 0.696 (0.316–1.534) | 0.369 | 0.109 | 0.550 (0.253–1.197) | 0.132 |
ADC, adenocarcinoma; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; STAS, spread through air spaces.
Table 3.
Cox proportional-hazards regression model for OS and DFS in patients with stage IB lung ADC in the primary cohort.
| Variables | STAS-negative |
STAS-positive |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS | OS | DFS | |||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| p | HR (95% CI) | p | p | HR (95% CI) | p | p | HR (95% CI) | p | p | HR (95% CI) | p | |
| Histologic Pattern | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.017 | <0.001 | 0.003 | ||||
| Lepidic predominant | 0.375 (0.196–0.720) | 0.003 | 0.389 (0.201–0.751) | 0.005 | 0.784 (0.409–1.503) | 0.464 | 0.816 (0.431–1.545) | 0.533 | ||||
| Acinar predominant | 0.421 (0.234–0.756) | 0.004 | 0.432 (0.239–0.780) | 0.005 | 0.825 (0.446–1.523) | 0.538 | 0.831 (0.443–1.558) | 0.563 | ||||
| Papillary predominant | 0.260 (0.117–0.577) | 0.001 | 0.750 (0.426–1.321) | 0.319 | 0.940 (0.507–1.741) | 0.844 | 0.945 (0.506–1.766) | 0.859 | ||||
| Micropapillary predominant | 1.607 (0.993–2.600) | 0.053 | 1.870 (1.154–3.030) | 0.011 | 1.831 (1.039–3.228) | 0.037 | 2.044 (1.157–3.609) | 0.014 | ||||
| Solid predominant | 1 | 1 | 1 | 1 | ||||||||
| Surgical Procedure (Lob versus Sublobar) | <0.001 | 0.482 (0.340–0.685) | <0.001 | <0.001 | 0.588 (0.422–0.819) | 0.002 | <0.001 | 0.476 (0.323–0.702) | <0.001 | <0.001 | 0.468 (0.319–0.688) | <0.001 |
| Adjuvant Chemotherapy (with versus without) | 0.002 | 0.559 (0.368–0.850) | 0.006 | <0.001 | 0.463 (0.309–0.693) | <0.001 | 0.042 | 0.604 (0.397–0.919) | 0.018 | 0.016 | 0.565 (0.372–0.858) | 0.007 |
ADC, adenocarcinoma; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; STAS, spread through air spaces.
Surgical procedures and ACT affect survival in stage I ADC/STAS-positive patients
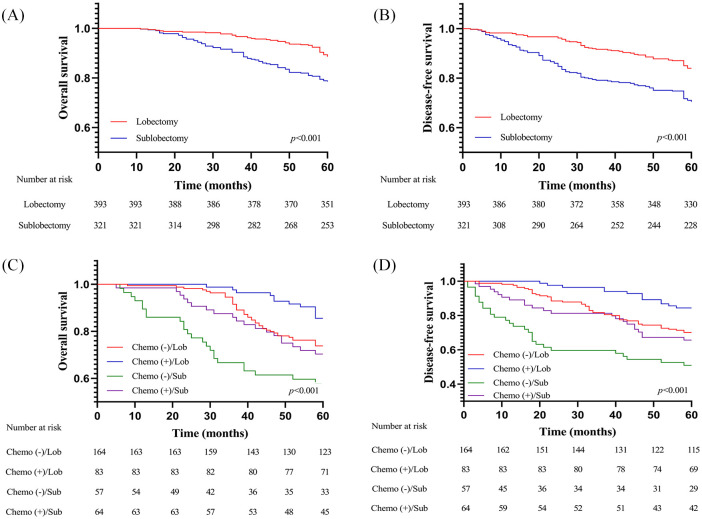
Among patients with stage IA ADC/STAS-positive, lobectomy was associated with better OS and DFS than SR, which was observed in both the primary cohort (Figure 2A,B) and in the validation cohort (Supplemental Figure S2A,B). In the multivariate analysis, we further identified lobectomy (OS: HR = 0. 509, 95% CI: 0.353–0.733; p < 0.001; DFS: HR = 0.470, 95% CI: 0.342–0.647; p < 0.001) as an independent predictor of favorable OS and DFS in stage IA ADC/STAS-positive (Table 2). However, ACT was not revealed as an independent factor for favorable survival in patients with stage IA ADC/STAS-positive (OS: HR = 0.696, 95% CI: 0.316–1.534; p = 0.369; DFS: HR = 0.550, 95% CI: 0.253–1.197; p = 0.132).
Figure 2.
(A) OS and (B) DFS of patients with stage IA ADC/STAS-positive undergoing different surgical procedures in the primary cohort; (C) OS and (D) DFS of patients with stage IB ADC/STAS-positive stratified by surgical procedures and administration of adjuvant chemotherapy in the primary cohort.
ADC, adenocarcinoma; DFS, disease-free survival; OS, overall survival; STAS, spread through air spaces.
Among patients with stage IB ADC/STAS-positive, lobectomy was also associated with advantages in OS and DFS compared with SR, while ACT was associated with additional survival benefits for different types of resection (Figure 2C,D). Notably, lobectomy plus ACT demonstrated the greatest superiority in OS and DFS compared with other treatment modalities. In the multivariate analysis, interestingly, both lobectomy and implementation of ACT were identified as independent predictors of favorable OS and DFS in stage IB ADC irrespective of STAS presence (Table 3). In other words, surgical procedures and ACT could effectively stratify patient survival in stage IB ADC/STAS-positive.
We further analyzed the types of recurrence in stage IB ADC/STAS-positive. The results revealed that surgical procedures and administration of ACT might be associated with increased risk of developing locoregional (p = 0.022) and distant (p < 0.001) recurrence (Supplemental Figure 3A,B). Interestingly, ACT was not associated with significantly decreased locoregional recurrence rate in patients receiving lobectomy (p = 0.184) and in those receiving SR (p = 0.348). Notably, lobectomy plus ACT showed significantly lower locoregional recurrence rate compared with SR plus ACT (p = 0.047). Meanwhile, patients receiving lobectomy alone shared a locoregional recurrence rate similar to those receiving SR plus ACT (p = 0.322) but had significantly lower locoregional recurrence rate than those receiving SR alone (p = 0.030). Besides, ACT was associated with decreased distant recurrence rate in patients undergoing SR (p = 0.035) and in those undergoing lobectomy (p = 0.023). It was also observed that lobectomy plus ACT had marginally lower distant recurrence rate than SR plus ACT (p = 0.061), while lobectomy alone resulted in significantly decreased distant recurrence rate compared with SR alone (p = 0.006).
Potential benefits of ACT in stage IA ADC/STAS-positive patients
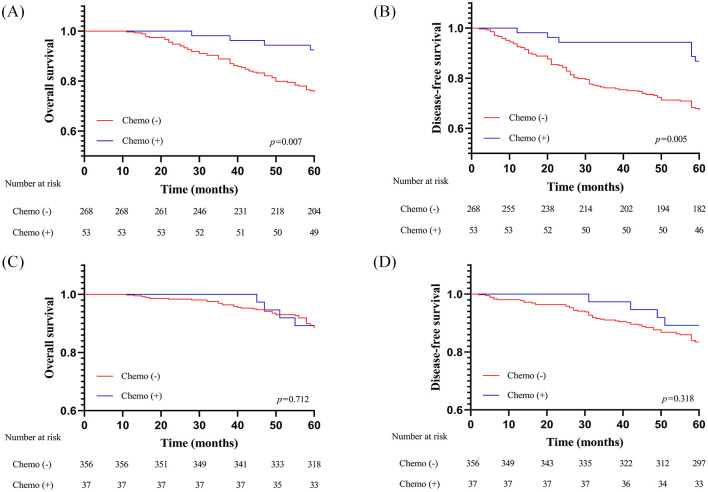
We further performed subgroup analysis on the survival of patients with stage IA ADC/STAS-positive who underwent SR in the primary cohort. As shown in Figure 3A,B, Kaplan–Meier curves revealed that patients with stage IA ADC/STAS-positive receiving ACT after SR had survival advantages compared with those without (OS: p = 0.007; DFS: p = 0.005). In multivariate analysis adjusted for age, sex, smoking history, T stage, and histologic subtype, ACT was also indicated as an independent factor for OS (HR = 0.787, 95% CI: 0.359–0.949; p = 0.034) and DFS (HR = 0.703, 95% CI: 0.330–0.904; p = 0.029) in patients who underwent SR for stage IA ADC/STAS-positive (Supplemental Table S4).
Figure 3.
OS and DFS survival of patients with stage IA ADC/STAS-positive receiving SR (A,B) and those receiving lobectomy (C,D) stratified by administration of adjuvant chemotherapy in the primary cohort.
ADC, adenocarcinoma; DFS, disease-free survival; OS, overall survival; SR, sublobar resection; STAS, spread through air spaces.
Regarding patients with stage IA ADC/STAS-positive receiving lobectomy, Kaplan–Meier survival analysis indicated that administration of ACT could not provide additional survival benefits (OS: p = 0.712; DFS: p = 0.318). Furthermore, it was observed that administration of ACT was not an independent predictor of more favorable OS (HR = 0.680, 95% CI: 0.234–1.976; p = 0.479) and DFS (HR = 0.546, 95% CI: 0.193–1.545; p = 0.254) in patients receiving lobectomy for stage IA ADC/STAS-positive in the multivariate analysis (Supplemental Table S5).
Discussion
Increasing evidence has consistently demonstrated that the presence of STAS in ADCs is associated with an increased risk of recurrence and a decreased rate of survival.10,13,14,19–21 However, no study has assessed the synergistic roles of surgical procedures and adjuvant treatment in stage I ADC patients with pathologically identified STAS. To the best of our knowledge, the present study is the first investigation to offer deep insights into the administration of ACT in stage I ADC/STAS-positive patients.
In our study, we initially confirmed that the presence of STAS remained a robust prognostic factor in both stage IA and IB cohorts. It was noteworthy that stage IA ADC/STAS-positive patients had similar OS and DFS as stage IB ADC/STAS-negative patients. Then, we demonstrated that surgical procedures and ACT could affect survival in stage I ADC/STAS-positive patients. More specifically, lobectomy was associated with better outcomes than SR in both stage IA and IB ADC/STAS-positive patients, while administration of ACT was an independent predictor of favorable survival only in stage IB ADC/STAS-positive patients. In terms of patients receiving SR for a stage IA ADC/STAS-positive diagnosis, ACT was revealed as an independent predictor of improved survival. Interestingly, administration of ACT did not bring additional survival benefits in stage IA ADC/STAS-positive patients receiving lobectomy.
In recent years, an increasing number of studies have assessed potential candidates with stage IA ADC who might benefit from ACT.22–25 It has been reported that ACT might improve outcomes after resection of stage IA ADC with lymphovascular invasion, which is another invasion pattern of ADC in addition to STAS. Liu et al. proposed that poor differentiation is an independent prognostic factor in pathological stage IA ADC after surgical resection.22 Recently, Wang et al. also confirmed the survival advantages of ACT for patients with micropapillary-predominant pattern in stage IA.24 Notably, STAS was found significantly associated with high-grade histological patterns including solid- and micropapillary-predominant subtypes in previous studies.10,26,27 Therefore, we further confirmed administration of ACT as a predictor of favorable OS in stage I/STAS-positive patients irrespective of micropapillary or solid component presence (Supplemental Table S6). Additionally, it has been revealed that STAS could be observed in residual lung segments after SR for stage IA disease.12 Taken together, it was reasonable to observe that ACT brought improved survival to stage IA ADC/STAS-positive patients receiving SR rather than those receiving lobectomy.
A number of studies have investigated different approaches to identify STAS in ADCs pre- and intraoperatively.13,19,28–31 To name just a few, Toyokawa et al. reported that the presence of notch and the absence of ground-glass opacity (GGO) were associated significantly with STAS in ADCs.29 Such findings were supported by Kim et al., who observed that STAS was also associated with central low attenuation, ill-defined opacity, air bronchogram, and large percentage of solid component.30 Our previous study proved that radiomics-based prediction using machine learning was also a promising approach to identify STAS preoperatively, which was supported by Zhuo et al. and Jiang et al.28,32 Recently, Suh et al. developed a stepwise flowchart for decision making on SR through the estimation of STAS consisting of both preoperative radiological features and intraoperative frozen pathology, which displayed good performance.33 In terms of frozen pathology, Eguchi et al. found that, in patients with T1 ADC, identifying STAS in frozen sections could be feasible with proved acceptable sensitivity and high specificity.13 Besides, it has been reported that intraoperative imprint cytology with the N–H classification for ADC was well correlated with the STAS status of the tumor.34 Therefore, mounting methods have emerged to allow the precise detection of STAS, which will be helpful in the decision-making process for surgical procedures.
We have to acknowledge some limitations of our study. First, the retrospective nature of our multicenter study might lead to selection and performance bias. Second, the number of STAS-positive patients was limited in the validation cohort, especially those receiving SR or ACT, which resulted in the unavailability of external validation of the potential benefits of ACT in stage IA ADC/STAS-positive cohorts. Third, there have been controversies as to whether any part of the free-floating tumor cell clusters identified as STAS are actually ex vivo artifacts. The most notable is the possibility that the tumor cell clusters can be spread through knife cuts made at the time of specimen processing, named as “spread through a knife surface” (STAKS).35 Although at least three tumor slices were observed under the microscope for each specimen, it was inevitable for us to include patients with STAKS rather than true STAS. By the way, we did not assess the relationship between STAS morphology and ACT benefit because of the limited number of STAS-positive patients who underwent ACT in both cohorts. Additionally, ACT decision and regimen selection were based on subjective preference rather than randomization.
In conclusion, the presence of STAS is correlated with poor prognosis in patients with stage I ADC. ACT is a favorable prognostic factor for stage IB ADC/STAS-positive patients. ACT improves outcomes in stage IA ADC/STAS-positive patients who underwent SR, whereas it provides no additional survival benefits for stage IA ADC/STAS-positive patients receiving lobectomy.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-1-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank the patients and the study staff of the five medical centers for their contributions to our research. We would like to thank Huikang Xie (Department of Pathology, Tongji University Affiliated Shanghai Pulmonary Hospital), Yongsheng Zhang (Department of Pathology, the Second Affiliated Hospital of Soochow University), Guojian Gu (the First People’s Hospital of Taicang), Guiyuan Gu (Hai’an Hospital Affiliated to Nantong University), and Xiang Jiang (Department of Pathology, Suzhou Kowloon Hospital Shanghai Jiaotong University School of Medicine) for their help with reviewing the slides.
Footnotes
Author contributions: Conception and design: DC, XW, FZ and RH;
Acquisition of data: DC, XW, QD, JS, FY, LS, YM.
Analysis and interpretation of data: DC, XW, FZ, RH and XX.
Drafting of the manuscript: DC, XW, FZ, RH, QD and XX.
Critical revision of the manuscript for important intellectual content: LS, YM, JS and FY.
Supervision and acquisition of the funding: CC and YC.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the projects from Suzhou Key Laboratory of Thoracic Oncology (SZS201907), Suzhou Key Discipline for Medicine (SZXK201803), Municipal Program of People’s Livelihood Science and Technology in Suzhou (SS2019061) and Jiangsu Key Research and Development Plan (Social Development) Project (BE2020653).
ORCID iD: Yongbing Chen  https://orcid.org/0000-0002-7595-092X
https://orcid.org/0000-0002-7595-092X
Availability of data and materials: Data is available upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Donglai Chen, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Xiaofan Wang, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Fuquan Zhang, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Ruoshuang Han, Department of Oncology, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Qifeng Ding, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Xuejun Xu, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Jian Shu, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China Department of Thoracic Surgery, Taicang Affiliated Hospital of Soochow University, The First People’s Hospital of Taicang, Taicang, China.
Fei Ye, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China Department of Thoracic Surgery, Hai’an Hospital Affiliated to Nantong University, Hai’an, China.
Li Shi, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Yiming Mao, Department of Thoracic Surgery, Suzhou Kowloon Hospital, Shanghai Jiaotong University School of Medicine, Suzhou, 215000, China.
Yongbing Chen, Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, 1055 Sanxiang Road, Gusu District, Suzhou, 215004, China.
Chang Chen, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, 200433, China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIa completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol 2017; 35: 2960–2974. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): non-small cell lung cancer, version 6. 2020. https://www.nccn.org/patients/guidelines/cancers.aspx#nsclc. (2020, accessed 15 June 2020)
- 4. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 5. Warth A, Muley T, Meister M, et al. The Novel Histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012; 30: 1438–1446. [DOI] [PubMed] [Google Scholar]
- 6. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg 2018; 155: 1227–1235e2. [DOI] [PubMed] [Google Scholar]
- 8. Ma M, She Y, Ren Y, et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J Thorac Dis 2018; 10: 5384–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adusumilli PS. Spread through alveolar spaces: an aerogenous invasion in pulmonary adenocarcinomas. J Thorac Cardiovasc Surg 2016; 152: 73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen D, Mao Y, Wen J, et al. Tumor spread through air spaces in non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg 2019; 108: 945–954. [DOI] [PubMed] [Google Scholar]
- 11. Wang S, Hao J, Qian C, et al. Tumor spread through air spaces is a survival predictor in non-small-cell lung cancer. Clin Lung Cancer 2019; 20: e584–e591. [DOI] [PubMed] [Google Scholar]
- 12. Ren Y, Xie H, Dai C, et al. Prognostic impact of tumor spread through air spaces in sublobar resection for 1a lung adenocarcinoma patients. Ann Surg Oncol 2019; 26: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 13. Eguchi T, Kameda K, Lu S, et al. Lobectomy is associated with better outcomes than sublobar resection in Spread Through Air Spaces (STAS)-positive T1 lung adenocarcinoma: a propensity score matched analysis. J Thorac Oncol. Epub ahead of print 19 September 2018. DOI: 10.1016/j.jtho.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai C, Xie H, Su H, et al. Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017; 12: 1052–1060. [DOI] [PubMed] [Google Scholar]
- 15. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 16. Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015; 10: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding Q, Chen D, Wang X, et al. Characterization of lung adenocarcinoma with a cribriform component reveals its association with spread through air spaces and poor outcomes. Lung Cancer 2019; 134: 238–244. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Chen D, Qiu X, et al. Relationship between MTA1 and spread through air space and their joint influence on prognosis of patients with stage I-III lung adenocarcinoma. Lung Cancer 2018; 124: 211–218. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, She Y, Wang T, et al. Radiomics-based prediction for tumour spread through air spaces in stage I lung adenocarcinoma using machine learning. Eur J Cardiothorac Surg 2020; 58: 51–58. [DOI] [PubMed] [Google Scholar]
- 20. Vaghjiani RG, Takahashi Y, Eguchi T, et al. Tumor spread through air spaces is a predictor of occult lymph node metastasis in clinical stage IA lung adenocarcinoma. J Thorac Oncol 2020; 15: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toki MI, Harrington K, Syrigos KN. The role of Spread Through Air Spaces (STAS) in lung adenocarcinoma prognosis and therapeutic decision making. Lung Cancer 2020; 146: 127–133. [DOI] [PubMed] [Google Scholar]
- 22. Liu CH, Peng YJ, Wang HH, et al. Heterogeneous prognosis and adjuvant chemotherapy in pathological stage I non-small cell lung cancer patients. Thorac Cancer 2015; 6: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodard GA, Wang SX, Kratz JR, et al. Adjuvant chemotherapy guided by molecular profiling and improved outcomes in early stage, non-small-cell lung cancer. Clin Lung Cancer 2018; 19: 58–64. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Yang J, Lu M. Micropapillary predominant lung adenocarcinoma in stage IA benefits from adjuvant chemotherapy. Ann Surg Oncol 2020; 27: 2051–2060. [DOI] [PubMed] [Google Scholar]
- 25. Wang S, Xu J, Wang R, et al. Adjuvant chemotherapy may improve prognosis after resection of stage I lung cancer with lymphovascular invasion. J Thorac Cardiovasc Surg 2018; 156: 2006–2015e2. [DOI] [PubMed] [Google Scholar]
- 26. Lee JS, Kim EK, Kim M, et al. Genetic and clinicopathologic characteristics of lung adenocarcinoma with tumor spread through air spaces. Lung Cancer 2018; 123: 121–126. [DOI] [PubMed] [Google Scholar]
- 27. Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol 2017; 12: 1788–1797. [DOI] [PubMed] [Google Scholar]
- 28. Jiang C, Luo Y, Yuan J, et al. CT-based radiomics and machine learning to predict spread through air space in lung adenocarcinoma. Eur Radiol 2020; 30: 4050–4057. [DOI] [PubMed] [Google Scholar]
- 29. Toyokawa G, Yamada Y, Tagawa T, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg 2018; 156: 1670–1676e4. [DOI] [PubMed] [Google Scholar]
- 30. Kim SK, Kim TJ, Chung MJ, et al. Lung adenocarcinoma: CT features associated with spread through air spaces. Radiology 2018; 289: 831–840. [DOI] [PubMed] [Google Scholar]
- 31. Walts AE, Marchevsky AM. Current evidence does not warrant frozen section evaluation for the presence of tumor spread through alveolar spaces. Arch Pathol Lab Med 2018; 142: 59–63. [DOI] [PubMed] [Google Scholar]
- 32. Zhuo Y, Feng M, Yang S, et al. Radiomics nomograms of tumors and peritumoral regions for the preoperative prediction of spread through air spaces in lung adenocarcinoma. Transl Oncol 2020; 13: 100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suh JW, Jeong YH, Cho A, et al. Stepwise flowchart for decision making on sublobar resection through the estimation of spread through air space in early stage lung cancer. Lung Cancer 2020; 142: 28–33. [DOI] [PubMed] [Google Scholar]
- 34. Kimura T, Nakamura H, Omura A, et al. Novel imprint cytological classification is correlated with tumor spread through air spaces in lung adenocarcinoma. Lung Cancer 2020; 148: 62–68. [DOI] [PubMed] [Google Scholar]
- 35. Blaauwgeers H, Flieder D, Warth A, et al. A prospective study of loose tissue fragments in non-small cell lung cancer resection specimens: an alternative view to “spread through air spaces”. Am J Surg Pathol 2017; 41: 1226–1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-1-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_1758835920978147 for Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study by Donglai Chen, Xiaofan Wang, Fuquan Zhang, Ruoshuang Han, Qifeng Ding, Xuejun Xu, Jian Shu, Fei Ye, Li Shi, Yiming Mao, Yongbing Chen and Chang Chen in Therapeutic Advances in Medical Oncology