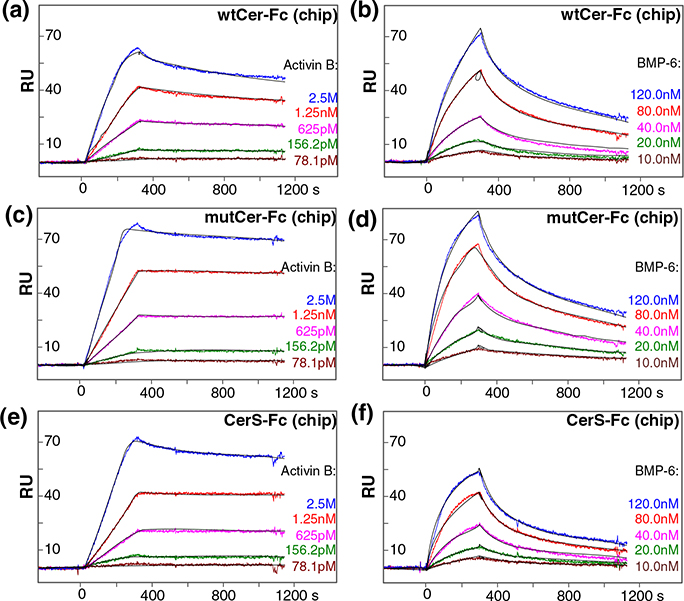
Fig. 3.
Cerberus ligand binding affinities. (a) Activin B–wtCer-Fc interaction. wtCer-Fc was immobilized on the SPR sensor chip and different concentrations of Activin B were injected as shown. The estimated Activin B–wtCer-Fc association constant (ka) is 2.3 × 106 M−1 s−1, the dissociation constant (kd) is 2.2 × 10−4 s−1, and the equilibrium dissociation constant (Kd) is 0.096 nM. (b) Activin B–mutCer-Fc interaction. mutCer-Fc was immobilized and Activin B was injected as shown. The estimated Activin B–mutCer-Fc association constant (ka) is 2.0 × 106 M−1 s−1, the dissociation constant (kd) is 3.1 × 10−5 s−1, and the equilibrium dissociation constant (Kd) is 0.016 nM. (c) Activin B–CerS-Fc interaction. CerS-Fc was immobilized and Activin B was injected. The estimated Activin B–CerS-Fc association constant (ka) is 1.2 × 106 M−1 s−1, the dissociation constant (kd) is 1.7 × 10−5 s−1, and the equilibrium dissociation constant (Kd) is 0.014 nM. (d) BMP-6–wtCer-Fc interaction. wtCer-Fc was immobilized and BMP-6 was injected. The estimated BMP-6–wtCer-Fc association constant (ka) is 5.5 × 104 M−1 s−1, the dissociation constant (kd) is 1.1 × 10−3 s−1, and the equilibrium dissociation constant (Kd) is 19 nM. (e) BMP-6–mutCer-Fc interaction. mutCer-Fc was immobilized and BMP-6 was injected. The estimated BMP-6–mutCer-Fc association constant (ka) is 6.9 × 104 M−1 s−1, the dissociation constant (kd) is 1.0 × 10−3 s−1, and the equilibrium dissociation constant (Kd) is 15 nM. (f) BMP-6–CerS-Fc interaction. CerS-Fc was immobilized BMP-6 was injected. The estimated BMP-6–CerS-Fc association constant (ka) is 8.2 × 104 M−1 s−1, the dissociation constant (kd) is 1.2 × 10−3 s−1, and the equilibrium dissociation constant (Kd) is 15 nM. (a–f) Fitted curves (black lines) are superimposed over all experimental curves.