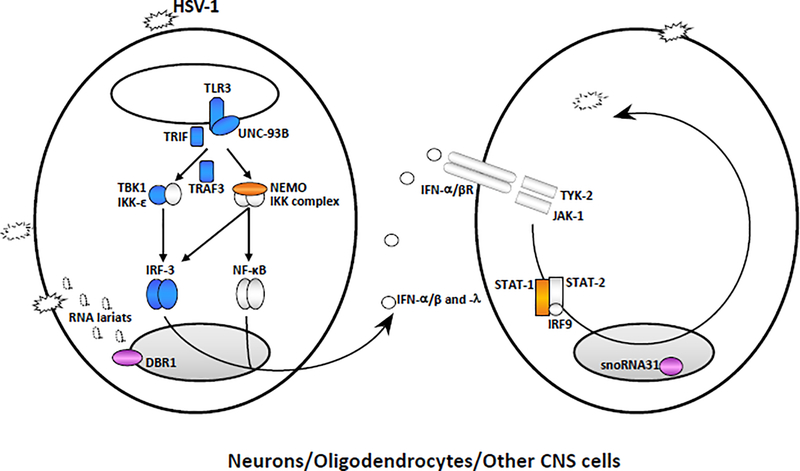
Figure 1. Inborn errors of immunity conferring predisposition to childhood HSE.
Monogenic inborn errors of the TLR3-IFN circuit or snoRNA31 confer susceptibility to forebrain HSE, whereas inborn errors of DBR1 underlie brainstem HSE. TLR3 signaling is initiated by the recognition of dsRNA, inducing activation of the IRF3 and NF-κB pathways via TRIF, leading to the production of IFN-α/β and/or IFN-λ. Mutations of six TLR3 signaling pathway genes (TLR3, UNC93B1, TRIF, TRAF3, TBK1, and IRF3, highlighted in blue) have been found in patients with forebrain HSE. Mutations of other two genes of the TLR3-IFN circuit (NEMO, STAT1, in orange) have been found in patients suffering from HSE together with mycobacterial disease. Impaired TLR3-dependent, IFN-mediated cortical neuron- and oligodendrocyte-autonomous anti-HSV-1 immunity may underlie the pathogenesis of HSE in patients with TLR3 pathway gene defects. SnoRNA31 deficiency impairs cortical neuron-intrinsic immunity to HSV-1. DBR1 is a protein that shuttles between the cell nucleus and cytoplasm. DBR1 deficiency leads to defective RNA lariat metabolism and impaired cell-intrinsic immunity to HSV-1, despite normal cellular responses to stimulation with TLR3 or IFN-α/β. The fine molecular and cellular mechanisms by which snoRNA31 or DBR1 (in violet) deficiencies cause forebrain and brainstem HSE, respectively, remain to be dissected in detail.