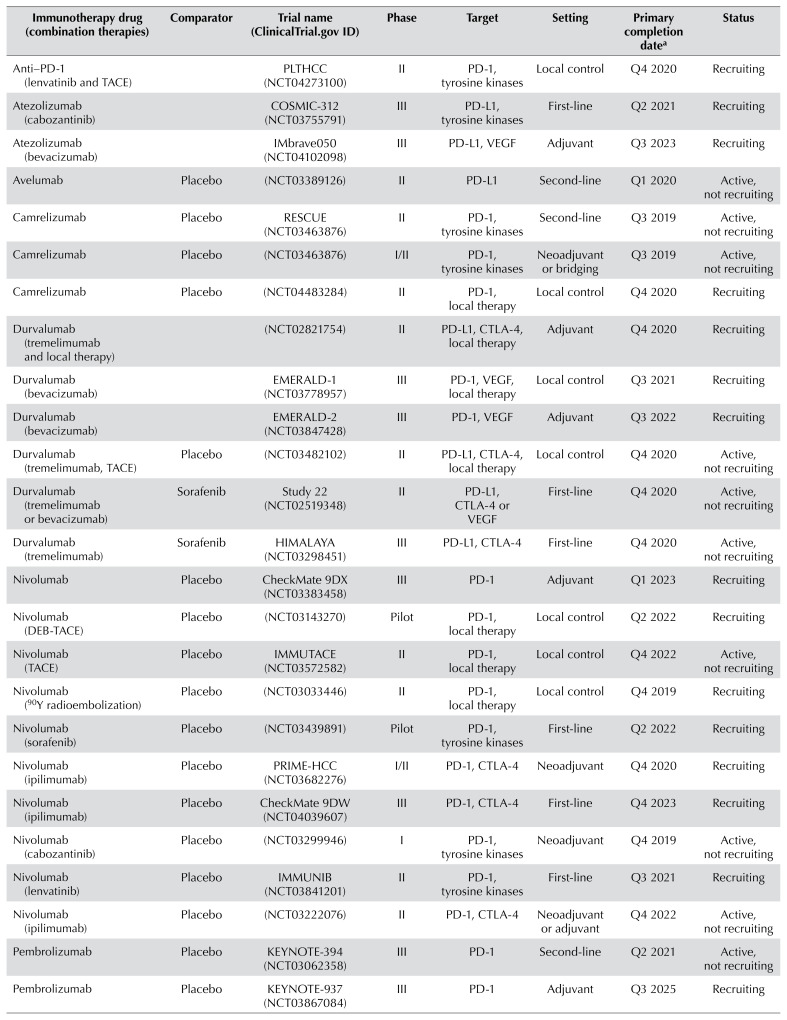
TABLE III.
Selection of ongoing clinical trials of immunotherapy in hepatocellular carcinoma
| Immunotherapy drug (combination therapies) | Comparator | Trial name (ClinicalTrials.gov ID) | Phase | Target | Setting | Primary completion datea | Status |
|---|---|---|---|---|---|---|---|
| Anti–PD-1 (lenvatinib and TACE) | PLTHCC (NCT04273100) | II | PD-1, tyrosine kinases | Local control | Q4 2020 | Recruiting | |
| Atezolizumab (cabozantinib) | COSMIC-312 (NCT03755791) | III | PD-L1, tyrosine kinases | First-line | Q2 2021 | Recruiting | |
| Atezolizumab (bevacizumab) | IMbrave050 (NCT04102098) | III | PD-L1, VEGF | Adjuvant | Q3 2023 | Recruiting | |
| Avelumab | Placebo | (NCT03389126) | II | PD-L1 | Second-line | Q1 2020 | Active, not recruiting |
| Camrelizumab | Placebo | RESCUE (NCT03463876) | II | PD-1, tyrosine kinases | Second-line | Q3 2019 | Active, not recruiting |
| Camrelizumab | Placebo | (NCT03463876) | I/II | PD-1, tyrosine kinases | Neoadjuvant or bridging | Q3 2019 | Active, not recruiting |
| Camrelizumab | Placebo | (NCT04483284) | II | PD-1, local therapy | Local control | Q4 2020 | Recruiting |
| Durvalumab (tremelimumab and local therapy) | (NCT02821754) | II | PD-L1, CTLA-4, local therapy | Adjuvant | Q4 2020 | Recruiting | |
| Durvalumab (bevacizumab) | EMERALD-1 (NCT03778957) | III | PD-1, VEGF, local therapy | Local control | Q3 2021 | Recruiting | |
| Durvalumab (bevacizumab) | EMERALD-2 (NCT03847428) | III | PD-1, VEGF | Adjuvant | Q3 2022 | Recruiting | |
| Durvalumab (tremelimumab, TACE) | Placebo | (NCT03482102) | II | PD-L1, CTLA-4, local therapy | Local control | Q4 2020 | Active, not recruiting |
| Durvalumab (tremelimumab or bevacizumab) | Sorafenib | Study 22 (NCT02519348) | II | PD-L1, CTLA-4 or VEGF | First-line | Q4 2020 | Active, not recruiting |
| Durvalumab (tremelimumab) | Sorafenib | HIMALAYA (NCT03298451) | III | PD-L1, CTLA-4 | First-line | Q4 2020 | Active, not recruiting |
| Nivolumab | Placebo | CheckMate 9DX (NCT03383458) | III | PD-1 | Adjuvant | Q1 2023 | Recruiting |
| Nivolumab (DEB-TACE) | Placebo | (NCT03143270) | Pilot | PD-1, local therapy | Local control | Q2 2022 | Recruiting |
| Nivolumab (TACE) | Placebo | IMMUTACE (NCT03572582) | II | PD-1, local therapy | Local control | Q4 2022 | Active, not recruiting |
| Nivolumab (90Y radioembolization) | Placebo | (NCT03033446) | II | PD-1, local therapy | Local control | Q4 2019 | Recruiting |
| Nivolumab (sorafenib) | Placebo | (NCT03439891) | Pilot | PD-1, tyrosine kinases | First-line | Q2 2022 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | PRIME-HCC (NCT03682276) | I/II | PD-1, CTLA-4 | Neoadjuvant | Q4 2020 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | CheckMate 9DW (NCT04039607) | III | PD-1, CTLA-4 | First-line | Q4 2023 | Recruiting |
| Nivolumab (cabozantinib) | Placebo | (NCT03299946) | I | PD-1, tyrosine kinases | Neoadjuvant | Q4 2019 | Active, not recruiting |
| Nivolumab (lenvatinib) | Placebo | IMMUNIB (NCT03841201) | II | PD-1, tyrosine kinases | First-line | Q3 2021 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | (NCT03222076) | II | PD-1, CTLA-4 | Neoadjuvant or adjuvant | Q4 2022 | Active, not recruiting |
| Pembrolizumab | Placebo | KEYNOTE-394 (NCT03062358) | III | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
| Pembrolizumab | Placebo | KEYNOTE-937 (NCT03867084) | III | PD-1 | Adjuvant | Q3 2025 | Recruiting |
| Pembrolizumab (lenvatinib) | Placebo | LEAP-002 (NCT03713593) | III | PD-1, tyrosine kinases | First-line | Q2 2022 | Active, not recruiting |
| Pembrolizumab (TACE) | Placebo | PETAL (NCT03397654) | IB | PD-1 | Local control | Q2 2020 | Recruiting |
| Pembrolizumab | Placebo | (NCT03419481) | II | PD-1 | First-line | Q3 2020 | Recruiting |
| Pembrolizumab (elbasvir/grazoprevir) | Placebo | (NCT02940496) | I/II | PD-1, HCV therapy | Second-line | Q4 2021 | Recruiting |
| Pembrolizumab (local therapy) | Placebo | IMMULAB (NCT03753659) | II | PD-1, local therapy | Local control | Q3 2022 | Recruiting |
| Tislelizumab | Placebo | RATIONALE-208 (NCT03419897) | II | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
| Tislelizumab | Sorafenib | RATIONALE-301 (NCT03412773) | III | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
Actual or estimated.
TACE = transarterial chemoembolization; VEGF = vascular endothelial growth factor; DEB-TACE = drug-eluting bead TACE.