Abstract
Following a request from the European Commission, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety of 31 compounds belonging to different chemical groups, when used as sensory additives in feed for all animal species. Twenty‐two out of the 31 compounds were tested in tolerance studies in chickens for fattening, piglets and cattle for fattening. For the remaining nine compounds, read across from structurally similar compounds was proposed. No adverse effects were observed in the tolerance studies at 10‐fold the intended level. The FEEDAP Panel concluded that the 22 compounds are safe for these species at the proposed use level and conclusions were extrapolated to all animal species for all the compounds except for α‐damascone [07.134]. In the absence of data that would allow the FEEDAP Panel to rule out the genotoxicity concern, the FEEDAP Panel cannot extend the conclusions for α‐damascone [07.134] to all animal species and cannot conclude on the safety for the consumer, the user and the environment. No safety concern would arise for the consumer from the use of the remaining 30 compounds up to the highest levels considered safe for target animals. The revised maximum safe levels for the 30 compounds are not expected to further impact on the previous conclusions on user safety. The concentrations considered safe for the target species are unlikely to have detrimental effects on the environment for all the compounds except β‐damascone [07.083] and (E)‐β‐damascone [07.224], for which in the absence of ecotoxicity data, the FEEDAP Panel cannot conclude on the safety for the terrestrial compartments. For the marine environment, the safe use level for 2‐methyl‐1‐phenylpropan‐2‐ol [02.035], α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224], phenethyl isovalerate [09.466], 4‐(p‐hydroxyphenyl) butan‐2‐one [07.055] and 2‐isopropyl‐4‐methylthiazole [15.026] is confirmed to be 0.05 mg/kg.
Keywords: sensory additives, flavourings, tolerance studies with mixture of flavourings, environment
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition and, in particular, Article 9 defined the term of the authorisation by the Commission.
The applicant, FEFANA asbl, is seeking a Community authorisation of Chemically defined flavourings as feed additives to be used as flavourings compounds for all animal species (Table 1).
Table 1.
Description of the substances
| Category of additive | Sensory additive |
| Functional group of additives | Flavouring compounds |
| Description |
Dodecanal Ethyl heptanoate Ethyl 2‐methylbutyrate Isopentyl acetate 3‐Methylbutyl 3‐methylbutyrate Hex‐2‐en‐1‐ol Hex‐2(trans)‐enal Allyl hexanoate Linalool 2‐Methyl‐1‐phenylpropan‐2‐ol alpha‐Ionone beta‐Damascone Nootkatone alpha‐Damascone Pentadecano‐1,15‐lactone 2‐Phenylethan‐1‐ol Phenethyl isovalerate 8‐Mercapto‐p‐menthan‐3‐one 4‐(p‐Hydroxyphenyl) butan‐2‐one 2‐Methoxynaphthalene 2‐Isopropyl‐4‐methylthiazole Valencene 2‐Methylpropionic acid 3‐Methylbutyl butyrate 2‐Methylbutyl acetate Allyl heptanoate beta‐Ionone 4‐(2,5,6,6‐Tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one beta‐Damascenone tr‐1‐(2,6,6‐Trimethyl‐1‐cyclohexen‐1‐yl)but‐2‐en‐1‐one p‐Menth‐1‐ene‐8‐thiol Belonging to different chemical groups (see Table 2) |
| Target animal category | All animal species |
| Applicant | FEFANA asbl |
| Type of request | New opinion |
The Panel on Additives and Products or Substances used in Animal Feed of the European Food Safety Authority (“Authority”), in its opinions on the safety and efficacy of the above‐mentioned additives (see Table 2), could not conclude on the safety of for the maximum levels proposed by the applicant.
Table 2.
Flavourings compounds under assessment, grouped according to the chemical group (CG) as defined in Commission Regulation (EC) No 1565/20002, with indication of the EU Flavour Information System (FLAVIS) number and the corresponding FEEDAP opinion (year)
| CG | Chemical group | Product (EU register name) | FLAVIS no | FEEDAP opinion |
|---|---|---|---|---|
| 01 | Straight‐chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes | Dodecanal | 05.011 | 2013 |
| Ethyl heptanoate | 09.093 | |||
| Ethyl 2‐methylbutyrate | 09.409 | |||
| 02 | Branched‐chain primary aliphatic alcohols/aldehydes/acids, acetal and esters | 2‐Methylpropionic acid | 08.006 | 2012e |
| Isopentyl acetate | 09.024 | |||
| 3‐Methylbutyl butyrate | 09.055 | |||
| 2‐Methylbutyl acetate | 09.286 | |||
| 3‐Methylbutyl 3‐methylbutyrate | 09.463 | |||
| 03 | a, ß‐Unsaturated (alkene or alkyne) straight‐chain and branched‐chain aliphatic primary alcohols/aldehydes/ acids, acetals and esters | Hex‐2‐en‐1‐ol | 02.020 | 2019b |
| Hex‐2(trans)‐enal | 05.073 | |||
| Allyl hexanoate | 09.244 | |||
| Allyl heptanoate | 09.097 | |||
| 06 | Aliphatic alcohols | Linalool | 02.013 | 2012f |
| 2‐Methyl‐1‐phenylpropan‐2‐ol | 02.035 | |||
| 08 | Secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols | α‐Ionone | 07.007 | 2016c |
| β‐Ionone | 07.008 | |||
| 4‐(2,5,6,6‐Tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one (referred as to α‐irone) | 07.011 | |||
| β‐Damascone | 07.083 | |||
| Nootkatone | 07.089 | |||
| β‐Damascenone | 07.108 | |||
| tr‐1‐(2,6,6‐Trimethyl‐1‐cyclohexen‐1‐yl)but‐2‐en‐1‐one (referred as to (E)‐β‐damascone) | 07.224 | |||
| α‐Damascone | 07.134 | 2020 | ||
| 09 | Primary aliphatic saturated or unsaturated alcohols/aldehydes/acids/ acetals/esters with a second primary, secondary or tertiary oxygenated functional group | Pentadecano‐1,15‐lactone | 10.004 | 2012d |
| 15 | Phenyl ethyl alcohols, phenylacetic acids, related esters, phenoxyacetic acids and related esters | 2‐Phenylethan‐1‐ol | 02.019 | 2012b |
| Phenethyl isovalerate | 09.466 | |||
| 20 | Aliphatic and aromatic mono‐ and dithiols and mono‐, di‐, tri‐ and polysulfides with or without additional oxygenated functional groups | 8‐Mercapto‐p‐menthan‐3‐one | 12.038 | 2019a |
| p‐Menth‐1‐ene‐8‐thiol | 12.085 | |||
| 21 | Aromatic ketones, secondary alcohols and related esters | 4‐(p‐Hydroxyphenyl) butan‐2‐one | 07.055 | 2016c |
| 26 | Aromatic ethers including anisole derivatives | 2‐Methoxynaphthalene | 04.074 | 2012c |
| 29 | Thiazoles, thiophene, thiazoline and thienyl derivatives | 2‐Isopropyl‐4‐methylthiazole | 15.026 | 4441, 2016b |
| 31 | Aliphatic and aromatic hydrocarbons and acetals containing saturated aldehydes | Valencene | 01.017 | 4339, 2016a |
The list of flavouring compounds together with the EU Flavour Information System (FLAVIS) number, the chemical group as defined in Commission Regulation (EC) No 1565/20002 and reference to the corresponding FEEDAP opinion is given in Table 2.
The Commission gave the possibility to the applicant to submit complementary information in order to complete the assessment and to allow a revision of Authority's opinion. The new data have been received on 29 November 2019.
In view of the above, the Commission asks the Authority to deliver a new opinion on the safety of the 31 compounds listed in Table 2 as feed additives for all animal species based on the additional data submitted by the applicant.
1.2. Additional information
In the context of the re‐evaluation of feed flavourings, the FEEDAP Panel issued 36 opinions dealing with 560 compounds. For about 35% of the compounds assessed, in the absence of data (tolerance studies and/or toxicological studies with the additives under assessment from which a no observed adverse effect level (NOAEL) could be derived) or because of the unsuitability of the available toxicological data, the FEEDAP Panel could not conclude on the safety for target animals of the compounds at the maximum use level proposed by the applicant. The FEEDAP Panel, however, was in each case able to identify a lower safe use level for all animal species, based on the available toxicological information or, more commonly, based on the application of the threshold of toxicological concern (TTC) approach. The FEEDAP Panel also concluded that no safety concern would arise for the consumer or for the environment from the use of these compounds at the identified safe levels in feed.
For a number of substances, the safe use level identified by the FEEDAP Panel was lower than that typically used in feed and, in some cases, considered by the industry to be too low to allow an effective use as flavouring. The European Commission gave the applicant the possibility to submit complementary information with the aim to demonstrate the safety of the proposed use levels and allow a revision of those EFSA opinions which the industry found restrictive. The applicant recognised that to provide tolerance or toxicological studies for each individual flavouring would not be feasible and would have required a very high number of animals. As an alternative, the applicant proposed the use of tolerance studies designed to test a number of flavouring compounds simultaneously in a mixture, using concentrations which reflected their commercial application and an overdose. The intention was then to conclude on a safe level in feed for each component of the mixture based on their concentration in the mixture and the outcome of the tolerance study.
Four different mixtures (characterised by different olfactory notes, i.e. milky‐vanilla, toasted cereal, herbal and TuttiFrutti) totalling 68 compounds have been designed to be tested in three major species, chickens for fattening, piglets and cattle for fattening, for a total of 12 tolerance trials. Based on the structural similarity within a chemical group, the applicant also proposed the extrapolation of the conclusions for some of the compounds tested in the tolerance trials to structurally similar compounds belonging to the same chemical group, giving an overall total of 133 compounds. Data on residues in manure samples (excreta from chickens and in faeces and urine from piglets and cattle for fattening) from animals fed the mixture of additives at the maximum recommended use level were also collected to be used in the assessment of the safety for the environment.
As the tolerance studies were started in October 2016, over a 3‐year planning, they were designed to follow the provisions present in the guidance on sensory additives (EFSA FEEDAP Panel, 2012a, 2012b, 2012c, 2012d, 2012e, 2012f), which was in place at that time. The FEEDAP Panel exceptionally accepts the approach.
This application deals with the results of tolerance studies made with one of the four mixtures tested and the implications for target animal safety, consumer safety and the environment.
This mixture covers 31 compounds under assessment, belonging to several chemical groups (CGs), namely CG 1, 2, 3, 6, 8, 9, 15, 20, 21, 26, 29 and 31, when used as a feed flavourings for all animal species which were assessed by the FEEDAP Panel (EFSA FEEDAP Panel, 2012b, 2012c, 2012d, 2012e, 2012f, 2013, 2016a, 2016b, 2016c, 2016d, 2019a, 2019b, 2020).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of supplementary information3 to previous applications on the same products.4
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in the previous assessment regarding the methods used for the control of the chemically defined groups in animal feed are valid and applicable for the current application.5
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety of 31 flavouring compounds belonging to different chemically defined groups is in line with the principles laid down in Regulation (EC) No 429/20086 and the relevant guidance documents: Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012a, 2012b, 2012c, 2012d, 2012e, 2012f), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008).
3. Assessment
The additives under assessment are 31 compounds belonging to several chemical groups, namely CG 1, 2, 3, 6, 8, 9, 15, 20, 21, 26 29 and 31, intended for use as sensory additives (functional group: flavouring compounds) in feed for all animal species.
In previous opinions of the FEEDAP Panel (EFSA FEEDAP Panel, 2012b, 2012c, 2012d, 2012e, 2012f, 2013, 2016a, 2016b, 2016c, 2016d, 2019a, 2019b, 2020), the 31 additives under assessment were fully characterised and evaluated for their safety and efficacy as flavouring substances. For one compound, α‐damascone [07.134], the FEEDAP Panel was unable to conclude on the safety because of the inconclusive assessment of its genotoxicity (EFSA FEEDAP Panel, 2020). For the remaining 30 compounds, the FEEDAP Panel could not conclude on the safety for target animals at the maximum use level proposed by the applicant. The Panel, however, was in each case able to identify a safe use level for all animal species, lower than the maximum proposed use level, based on the available toxicological information or, more commonly, based on the application of the TTC approach. The Panel also concluded that no safety concern would arise for the consumer or the environment from the use of these compounds at the identified safe levels in feed but did not conclude at the maximum use level proposed by the applicant.
The applicant has provided new data to address the limitations previously identified regarding the safety for the target species and the safety for the environment. The new data submitted consist of tolerance studies in chickens for fattening, piglets and cattle for fattening, performed with a mixture of the 22 flavourings under assessment. Data on residues in manure samples (excreta from chickens and in faeces and urine from piglets and cattle for fattening) from animals fed the mixture of additives at the maximum recommended use level were also collected to allow the FEEDAP Panel to review its assessment of the safety for the environment. For the remaining nine compounds under assessment, which were not tested in the tolerance trials, the applicant proposed to extrapolate the conclusions for structurally similar compounds tested in the tolerance studies.
No new data were submitted on the safety for the user that would allow the FEEDAP Panel to change its previous conclusion.
3.1. Conditions of use
The maximum recommended levels proposed by the applicant for each compound tested in the mixture of flavourings are shown in Table 3 (referring to onefold level). The conditions of use for the remaining nine compounds are summarised in Table 4 (Section 3.2.1.6).
Table 3.
Individual components of the mixture and intended dosages tested in tolerance trials
| CG | EU register name | FLAVIS no | 1× MRD | 3× MRD | 10× MRD |
|---|---|---|---|---|---|
| mg/kg complete feed | |||||
| 01 | Dodecanal | 05.011 | 5 | 15 | 50 |
| 01 | Ethyl heptanoate | 09.033 | 31.8 | 95.4 | 318 |
| 01 | Ethyl 2‐methylbutyrate | 09.409 | 25 | 75 | 250 |
| 02 | Isopentyl acetate | 09.024 | 125 | 375 | 1,250 |
| 02 | 3‐Methylbutyl 3‐methylbutyrate | 09.463 | 25 | 75 | 250 |
| 03 | Hex‐2‐en‐1‐ol | 02.020 | 5 | 15 | 50 |
| 03 | Hex‐2(trans)‐enal | 05.073 | 5 | 15 | 50 |
| 03 | Allyl hexanoate | 09.244 | 5 | 15 | 50 |
| 06 | Linalool | 02.013 | 30 | 90 | 300 |
| 06 | 2‐Methyl‐1‐phenylpropan‐2‐ol | 02.035 | 5 | 15 | 50 |
| 08 | α‐Ionone | 07.007 | 25 | 75 | 250 |
| 08 | β‐Damascone | 07.083 | 5 | 15 | 50 |
| 08 | Nootkatone | 07.089 | 5 | 15 | 50 |
| 08 | α‐Damascone | 07.134 | 5 | 15 | 50 |
| 09 | Pentadecano‐1,15‐lactone | 10.004 | 10 | 30 | 100 |
| 15 | 2‐Phenylethan‐1‐ol | 02.019 | 25 | 75 | 125 |
| 15 | Phenethyl isovalerate | 09.466 | 30 | 90 | 300 |
| 20 | 8‐Mercapto‐p‐menthan‐3‐one | 12.038 | 0.5 | 1.5 | 5 |
| 21 | 4‐(p‐Hydroxyphenyl) butan‐2‐one | 07.055 | 25 | 75 | 250 |
| 26 | 2‐Methoxynaphthalene | 04.074 | 1.2 | 3.6 | 12 |
| 29 | 2‐Isopropyl‐4‐methylthiazole | 15.026 | 1.5 | 4.5 | 15 |
| 31 | Valencene | 01.017 | 5 | 15 | 50 |
EU: European Union; FLAVIS Number: EU Flavour Information System numbers; MRD: maximum recommended dose.
Table 4.
Conditions of use for the nine compounds not tested in the tolerance trials
| CG | Product (EU register name) | FLAVIS no | All animal species (mg/kg) |
|---|---|---|---|
| 02 | 2‐Methylpropionic acid | 08.006 | 25 |
| 3‐Methylbutyl butyrate | 09.055 | 25 | |
| 2‐Methylbutyl acetate | 09.286 | 25 | |
| 03 | Allyl heptanoate | 09.097 | 5 |
| 08 | β‐Ionone | 07.008 | 25 |
| α‐Irone | 07.011 | 5 | |
| β‐Damascenone | 07.108 | 5 | |
| (E)‐β‐Damascone | 07.224 | 5 | |
| 20 | p‐Menth‐1‐ene‐8‐thiol | 12.085 | 0.05 |
3.2. Safety
3.2.1. Safety for the target species
3.2.1.1. Test item and feed preparation
The mixture tested in tolerance studies is named ‘TuttiFrutti’ (M2) and includes 22 flavouring compounds belonging to several chemical groups. The individual components of the mixture, their FLAVIS numbers, the maximum recommended dose (MRD, 1×) proposed by the applicant and the two overdoses tested, 3× MRD or 10× MRD per kg complete feed, are described in Table 3.
■■■■■
Homogeneity of the test product was tested on 10× MRD samples at 2‐week interval (day 1, 14 and 28), taking 10 individual subsamples by monitoring linalool, a compound with one of the highest recoveries, as a marker. The coefficient of variation ranged between 2.4% and 5.4% in poultry feed, between 2.5% and 3.3% in feed for piglets and between 6.2 and 9.4% in feed for cattle for fattening.
3.2.1.2. Tolerance study in chickens for fattening
A total of 736 1‐day‐old male chickens for fattening (Ross 308) were distributed to 32 pens in groups of 23 animals and allocated to four dietary treatments (eight replicates per treatment), blocking applied depending on the situation of the pen in the room location. Two basal diets (starter (up to day 14) and grower (from day 14 to 36)) based on maize and soya bean meal were either not supplemented (control) or supplemented with the mixture (M2) to provide 1× MRD, 3× MRD or 10× MRD per kg feed (confirmed by analysis). The test mixture was added daily to the basal diet. Feed from the previous day was removed from the feeder in each pen and weighed. Diets were offered in mash form until day 36 of life. Diets contained coccidiostats for the whole duration of the study.
Mortality and health status were checked daily and dead animals were necropsied. Animals were weighed on days 1, 14 and 35 (pen basis), feed intake was registered per pen and feed to gain ratio was calculated. Blood samples were taken from two birds per pen (one on day 35 and the other one on day 36) for haematology7 and blood biochemistry8 (the birds were randomly selected at the beginning of the study). The basic study design was a randomised complete block design of four dietary treatments allocated in eight blocks, with pen location as block criteria. An analysis of variance (ANOVA) was done with the data (pen basis, individual for the blood parameters) and considering the treatment as the main effect. Group means were compared with Tukey test. The significance level was set at 0.05.
The birds were in general good health throughout the study (mortality range: 1.1–2.7%, not statistically different between treatments). The feed intake and final body weight of the animals were lower (20%) than the ones expected for the genotype of birds used but this could be due partly to the use of mash feed.
The chickens in the control group showed final body weight of 2,090 g, average body weight gain 58.3 g/day and a feed conversion ratio of 1.47, no significant different with the other treatments. Chickens receiving M2 at 10‐fold of the MRD had lower final body weight and average daily weight gain (2,043 g and 57.1 g/day) and average daily feed intake (85.0 g/day) (p < 0.05) relative to chickens receiving M2 at the onefold of the MRD (2,121 g, 59.3 g/day and 87.6 g/day), but not relative to control or M2 at threefold MRD treatments. These effects were not treatment related and considered of small or little biological relevance.
Overall, no significant changes in blood haematological or serum biochemical parameters were observed when feeding birds with onefold, threefold or 10‐fold of the MRD of the premixture of flavourings M2.
The FEEDAP Panel concludes that the additive is safe under the proposed conditions of use with a margin of safety of 10.
3.2.1.3. Tolerance study in weaned piglets
A total of 144 Piétrain × (Landrace × Large White) weaned piglets of 33 days of age, half females and half males, with an initial body weight of 8.6 kg, were distributed, according to a randomised complete block design, to 36 pens each containing four animals (two males and two females). From day 7 of life to day 7 post weaning (pre‐experimental phase), piglets received a commercial medicated feed containing zinc oxide and, on the day of weaning, all animals were treated with a single dose of tulathromycin. Piglets were assigned, on the basis of initial body weight and pen location, to four dietary treatments (nine replicates per treatment). Two basal diets (pre‐starter, up to day 14 of trial), mainly based on maize and soya bean meal, were either not supplemented (control) or supplemented with the mixture (M2) to provide: 1× MRD, 3× MRD or 10× MRD per kg feed (confirmed by analysis). Feed was offered on ad libitum basis in mash form for 42 days.
Mortality and health status were checked daily. Piglets were individually weighed on days 1, 14 and 42 of trial. Feed intake was registered per pen and average daily gain, average daily feed intake and feed to gain ratio were calculated. At the end of the experiment (day 42 of trial), blood samples were taken from two piglets per pen (one male and one female) for haematology9 and blood biochemistry.10 The experimental unit was the pen for production traits and the individual for blood parameters. All data were analysed by using the generalised linear model (GLM) procedure of SAS. The treatment and the block were the main effects for production traits; the treatment, the block and the sex were the main effects for blood parameters. Tukey's test was used as post hoc analysis. The significance level was set at p < 0.05.
The health status of the piglets was good throughout the study. Three animals died in the threefold group (enteritis) and one animal was culled in the onefold group. There were no significant differences between the treatments on the performance of the weaned piglets (mean body weight 30.2 kg, daily feed intake 812 g and feed to gain ratio 1.58). As concerns blood analyses, red blood cells count was lowered, although differences in mean values were not significant, in treated groups when compared to control animals. Significant differences were noted for alkaline phosphatase (higher in threefold vs. onefold), calcium (higher in control vs. onefold and threefold), C‐reactive protein (higher in onefold vs. control) and creatinine (higher in control vs. 10‐fold). Most differences were not dose‐related, and all values were within the reference intervals provided.
The FEEDAP Panel concludes that the additive is safe under the proposed conditions of use with a margin of safety of 10.
3.2.1.4. Tolerance study in cattle for fattening
A total of 24 bulls (Holstein, 300–350 kg body weight) were used for the study. The bulls were individually housed in pens and the four dietary treatments were allocated considering the body weight of the animals (six replicates per treatment) in a random complete block design. Before the start of the experimental phase, the bulls received a common mash concentrate for 14–28 days to collect basal data (blood samples, body weight and feed intake). From the start of the study, the animals were fed a test concentrate and straw. The test concentrate was based on maize meal, barley grain meal, maize gluten feed and wheat middlings and was either not supplemented (control) or supplemented with the mixture (M2) to provide 1× MRD, 3× MRD or 10× MRD per kg feed (confirmed by analysis). Feed was prepared daily and the animals had free access to the mash concentrate and to straw in two separate feeders. Feed from the previous day was removed from the feeder in each pen and weighed. Water was offered ad libitum in each pen. The duration of the study was 42 days. Mortality and health status were checked every day. Animals were weighed on days 1, 21 and 42, while feed intake was registered daily for concentrate and weekly for straw; feed to gain ratio was calculated. Blood samples were taken on day 1 and day 42 from all animals for haematology11 and blood biochemistry.12 An ANOVA was carried out with the pen as the experimental unit. Performance data were analysed using a linear mixed‐effects model with repeated measurements. The model included the fixed effects of treatment, time and the interaction between them. Initial body weight was used as a covariate. Blood parameters were analysed as above, but for blood parameters of day 1, the model without repeated measures was used. The significance level was set at 0.05.
The general health of the animals was good throughout the study and no animals died. For the overall period, there were no statistically significant differences in body weight, average daily gain, feed intake (concentrate and straw) or feed to gain ratio among treatments. Regarding the blood haematology and biochemistry data, no differences were observed among treatments.
The study showed no negative effects when the additive was added up to 10‐fold of the MRD in the concentrate. Considering the intake of straw, the levels tested would correspond to 0.86, 2.5 and 8.4× the MDR. As the intake of concentrate was about 85% of the total dry matter intake of the animals, the real exposure to the additive was lower than the one intended in the conditions of use.
Consequently, the FEEDAP Panel concludes that the additive is safe under the proposed conditions of use with a margin of safety of at least at 8.5.
3.2.1.5. Conclusions on the safety for the target species for the compounds tested in the tolerance studies
Based on the tolerance studies in chickens for fattening, piglets and cattle for fattening in which no adverse effects were seen at intended 10‐fold overdose, the FEEDAP Panel considers that the 22 compounds are safe for these species at the proposed use level.
As the margin of safety is similar in all species, the conclusions are extrapolated to all animal species for all the compounds, except for α‐damascone [07.134].
Because of the previous inconclusive assessment of its genotoxicity, the Panel cannot extend the conclusions for α‐damascone [07.134] to all animal species.
3.2.1.6. Extrapolation of the conclusions of the tolerance studies
For the remaining nine compounds not tested in the tolerance trials, namely 2‐methylpropionic acid [08.006], 3‐methylbutyl butyrate [09.055], 2‐methylbutyl acetate [09.286], allyl heptanoate [09.097], β‐ionone [07.008], 4‐(2,5,6,6‐tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one (herein referred as to α‐irone [07.011]), β‐damascenone [07.108], tr‐1‐(2,6,6‐trimethyl‐1‐cyclohexen‐1‐yl)but‐2‐en‐1‐one (herein referred as to (E)‐β‐damascone [07.224]) and p‐menth‐1‐ene‐8‐thiol [12.085], the applicant proposed to extrapolate the conclusions for structurally similar compounds tested in the tolerance studies and belonging to the same chemical group.
The proposed conditions of use for the nine compounds candidate for read across are summarised in Table 4.
Read across has been widely applied in the risk assessment of food and feed flavourings. Based on considerations related to structural and metabolic similarities, flavourings are grouped into chemical groups as defined in Annex I of Regulation (EC) No 1565/2000 and structural groups named Flavouring Group Evaluation (FGE). According to the guidance on the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012a, 2012b, 2012c, 2012d, 2012e, 2012f), ‘The conclusions obtained for an individual flavouring may be extended to other flavourings belonging to the same structural group (e.g., an FGE)’.
The application of read across within a chemical group is applied on a case by case basis, considering the structural features, the physico‐chemical properties and the expected reactivity of the compounds under assessment, as discussed in the paragraphs below.
Chemical group 2
The applicant proposed to read across from isopentyl acetate [09.024] to 2‐methylpropionic acid [08.006], 3‐methylbutyl butyrate [09.055] and 2‐methylbutyl acetate [09.286]. The FEEDAP Panel considers that the proposal for read across is justified by the structural similarity among the compounds and is further supported by the similarity with another compound tested in the tolerance trial, 3‐methylbutyl 3‐methylbutyrate [09.463]. The chemical structures of the compounds belonging to CG 2 are shown in Figure 1.
Figure 1.
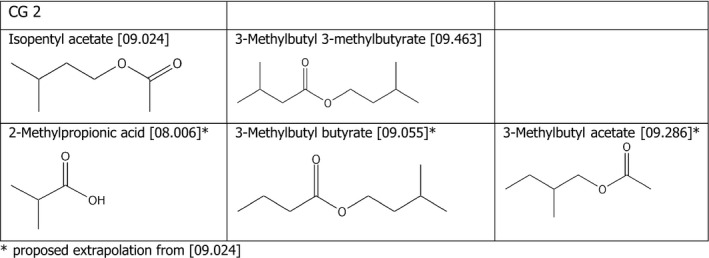
Chemical structures and FLAVIS number of the compounds belonging to chemical group 2 for which read across is proposed
Considering that no adverse effects were observed for isopentyl acetate [09.024] when tested in the tolerance studies in chickens, piglets and cattle for fattening up to 1,250 mg/kg and for 3‐methylbutyl 3‐methylbutyrate when tested up to 250 mg/kg feed, and considering the structural similarity of the compounds tested with the candidates for read across, the FEEDAP Panel concludes that the use of 2‐methylpropionic acid [08.006], 3‐methylbutyl butyrate [09.055] and 2‐methylbutyl acetate [09.286] at 25 mg/kg complete feed is safe for all animal species.
Chemical group 3
The applicant proposed to read across from allyl hexanoate [09.244] to allyl heptanoate [09.097]. The FEEDAP Panel considers that the proposal for read across is justified by the structural similarity between the two compounds, as shown in Figure 2.
Figure 2.
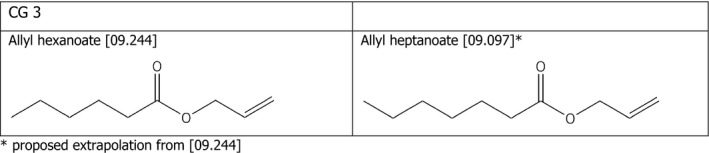
Chemical structures and FLAVIS number of the compounds belonging to chemical group 3 for which read across is proposed
Considering that no adverse effects were observed for allyl hexanoate [09.244] when tested up to 50 mg/kg in the tolerance studies in chickens, piglets and cattle for fattening, and considering the structural similarity between the two compounds, the FEEDAP Panel concludes that the use of allyl heptanoate [09.097] at 5 mg/kg complete feed is safe for all animal species.
Chemical Group 8
The applicant proposed to read across from α‐ionone [07.007] to β‐ionone [07.008] and α‐irone [07.011] (Figure 4). The FEEDAP Panel notes that the reactivity of the α,β‐unsaturated ketone moiety is influenced by the system of conjugated double bonds. To this regard, the compound tested in the tolerance trial (α‐ionone) shares the same features with α‐irone [07.011], but not with β‐ionone [07.008], which has a more extended conjugated system.
Figure 4.
Chemical structures and FLAVIS number of the compounds belonging to chemical group 20 for which read across is proposed
Therefore, the FEEDAP Panel considers that the results of the tolerance study for α‐ionone [07.007] cannot be extrapolated to β‐ionone [07.008]. For β‐ionone, the FEEDAP Panel retains its previous conclusion that the additive is safe at 5 mg/kg complete feed for salmonids, veal calves and dogs and at 1 mg/kg complete feed for the remaining target species.
For α‐irone [07.011], considering that no adverse effects were observed for α‐ionone [07.007] when tested in the tolerance studies in chickens, piglets and cattle for fattening up to 250 mg/kg, and considering the structural similarity between the two compounds, the FEEDAP Panel concludes that the use of α‐irone [09.097] at 5 mg/kg complete feed is safe for all animal species.
The applicant also proposed to read across from β‐damascone [07.083] to β‐damascenone [07.108] and (E)‐β‐damascone [07.224]. The FEEDAP Panel notes that the three compounds have differences either in the system of conjugated bonds (more expanded for β‐damascenone [07.018] than β‐damascone [07.083]) or in the geometric configuration β‐damascone [07.083] has a cis‐configuration and the others have a trans‐configuration. Since the reactivity of the α,β‐unsaturated ketone moiety is influenced by the system of conjugated bonds, the results of the tolerance study for β‐damascone [07.083] cannot be extrapolated to β‐damascenone [07.108], which has a more extended conjugated system. For β‐damascenone [07.108], the FEEDAP Panel retains the previous conclusions that the additive is safe at 1.5 mg/kg complete feed for cattle for fattening, salmonids and non‐food producing animals and at 1.0 mg/kg complete feed for pigs and poultry (EFSA FEEDAP Panel, 2016c).
Despite the differences in the geometric configuration of the conjugated double bond, the FEEDAP Panel considers that the trans‐isomer (E)‐β‐damascone [07.224] is expected to be less reactive than the cis‐isomer, which is less sterically hindered. Therefore, the result of the tolerance study for β‐damascone [07.083] can be extrapolated to the less reactive trans‐isomer (E)‐β‐damascone [07.224]. Considering that no adverse effects were observed for β‐damascone [07.083] when tested in the tolerance studies in chickens, piglets and cattle for fattening up to 50 mg/kg, and considering the structural similarity between the two compounds, the FEEDAP Panel concludes that the use of (E)‐β‐damascone [07.224], at 5 mg/kg complete feed is safe for all animal species (Figure 3).
Figure 3.
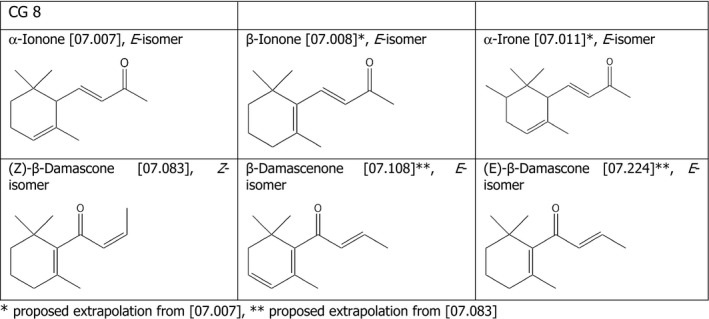
Chemical structures and FLAVIS number of the compounds belonging to chemical group 8 for which read across is proposed
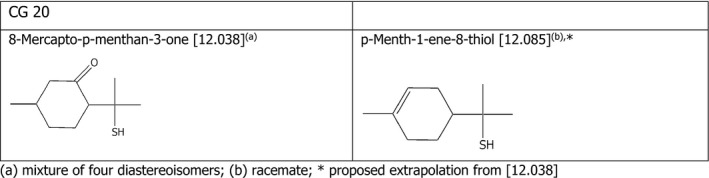
Chemical Group 20
The applicant proposed to read across from 8‐mercapto‐p‐menthan‐3‐one [12.038] to p‐menth‐1‐ene‐8‐thiol [12.085]. The chemical structures of the compounds belonging to CG 20 are shown in Figure 4.
8‐Mercapto‐p‐menthan‐3‐one [12.038] is an oxygenated compound (a cyclic ketone) whereas p‐menth‐1‐ene‐8‐thiol [12.085] has a double bond in the 6‐atom ring, whose reactivity is decreased by the substitution with a methyl group. The FEEDAP Panel notes that although the two thiols show some differences in their structure, these differences are not expected to influence the reactivity of the thiol, as S‐oxidation is the major metabolic pathway for all thiols, including oxygenated derivatives (see CG 20 opinion, EFSA FEEDAP Panel 2013). In addition, the proposed use levels are low, and equal or close to the safe level in feed calculated for Cramer class II compounds. Therefore, the FEEDAP Panel considers that the proposal for read across is justified.
Considering that no adverse effects were observed for 8‐mercapto‐p‐menthan‐3‐one [12.038] when tested in the tolerance studies in chickens, piglets and cattle for fattening up to 5 mg/kg, and considering the above, the FEEDAP Panel concludes that the use of p‐menth‐1‐ene‐8‐thiol [12.085] is safe at 0.5 mg/kg complete feed for all animal species.
3.2.1.7. Conclusions on safety for the target species
Based on the results of the tolerance studies in chickens for fattening, piglets and cattle for fattening, the FEEDAP Panel concludes that α‐damascone [07.134] is tolerated by these species at the maximum proposed use level of 5 mg/kg complete feed. Because of the inconclusive assessment of its genotoxicity, the Panel cannot extend the conclusions for α‐damascone [07.134] to all animal species.
The conclusions of the FEEDAP Panel on the maximum safe concentration of the 31 compounds in complete feed for all animal species are summarised in Table 5.
Table 5.
Maximum safe concentration in feed (mg/kg) for all animal species for the 31 compounds belonging to different chemical groups
| CG | Product (EU register name) | FLAVIS no | All animal species (mg/kg complete feed) |
|---|---|---|---|
| 01 | Dodecanal | 05.011 | 5 |
| Ethyl heptanoate | 09.093 | 31.8 | |
| Ethyl 2‐methylbutyrate | 09.409 | 25 | |
| 02 | 2‐Methylpropionic acid | 08.006 | 25 |
| Isopentyl acetate | 09.024 | 125 | |
| 3‐Methylbutyl butyrate | 09.055 | 25 | |
| 2‐Methylbutyl acetate | 09.286 | 25 | |
| 3‐Methylbutyl 3‐methylbutyrate | 09.463 | 25 | |
| 03 | Hex‐2‐en‐1‐ol | 02.020 | 5 |
| Hex‐2(trans)‐enal | 05.073 | 5 | |
| Allyl hexanoate | 09.244 | 5 | |
| Allyl heptanoate | 09.097 | 5 | |
| 06 | Linalool | 02.013 | 30 |
| 2‐Methyl‐1‐phenylpropan‐2‐ol | 02.035 | 5 | |
| 08 | α‐Ionone | 07.007 | 25 |
| β‐Ionone | 07.008 | 1–5* | |
| α‐Irone | 07.011 | 5 | |
| β‐Damascone | 07.083 | 5 | |
| Nootkatone | 07.089 | 5 | |
| β‐Damascenone | 07.108 | 1.0–1.5** | |
| (E)‐β‐Damascone | 07.224 | 5 | |
| α‐Damascone | 07.134 | 5*** | |
| 09 | Pentadecano‐1,15‐lactone | 10.004 | 10 |
| 15 | 2‐Phenylethan‐1‐ol | 02.019 | 25 |
| Phenethyl isovalerate | 09.466 | 30 | |
| 20 | 8‐Mercapto‐p‐menthan‐3‐one | 12.038 | 0.5 |
| p‐Menth‐1‐ene‐8‐thiol | 12.085 | 0.5 | |
| 21 | 4‐(p‐Hydroxyphenyl) butan‐2‐one | 07.055 | 25 |
| 26 | 2‐Methoxynaphthalene | 04.074 | 1.2 |
| 29 | 2‐Isopropyl‐4‐methylthiazole | 15.026 | 1.5 |
| 31 | Valencene | 01.017 | 5 |
Safe at 5 mg/kg feed for salmonids, veal calves and dogs, and at 1 mg/kg feed for the remaining species.
Safe at 1.5 mg/kg for cattle for fattening, salmonids and non‐food producing animals, and 1.0 mg/kg for pigs and poultry.
Safe at 5 mg/kg complete feed for chickens for fattening, piglets and cattle for fattening.
3.2.2. Safety for the consumer
In its previous opinion, the FEEDAP Panel was unable to conclude on the safety of α‐damascone [07.134] because of the inconclusive assessment of its genotoxicity (EFSA FEEDAP Panel, 2020). In the current application, no new data were submitted that would allow the FEEDAP Panel to rule out the genotoxicity concern for α‐damascone. In the absence of residue data in tissues of animals fed the additive at the use levels considered safe for the target species, the FEEDAP Panel is unable to conclude on the safety for the consumer for this compound.
The safety for the consumer of the remaining 30 compounds used as food flavours has been already assessed by JECFA and EFSA as described in the former opinions of the FEEDAP Panel (EFSA FEEDAP Panel 2012b,c,d,e,f, 2013, 2016a,b,c,d, 2019a,b). All compounds are currently authorised in the EU as food flavourings without limitations.
Although deposition and residue studies of the compounds in farm animals are not available, the FEEDAP Panel considers that the use of these flavourings in animal feed would not appreciably increase the human exposure to these compounds. This is based on the expected extensive metabolism and excretion in target animals.
Consequently, no safety concern would arise for the consumer from the use of these 30 compounds up to the highest levels considered safe for target animals.
3.2.3. Safety for the user
Regarding the safety for the user, in its previous assessments, the FEEDAP Panel concluded that the additives should be considered as irritant to skin and eyes and as potential skin and respiratory sensitisers in susceptible individuals. For hex‐2‐en‐1‐ol [02.020], hex‐2(trans)‐enal [05.073], allyl hexanoate [09.244] and allyl heptanoate [09.097] in CG 3, in the absence of studies to assess the safety for the user, the FEEDAP Panel cannot conclude on the safety for the users when handling the additives (EFSA FEEDAP Panel, 2019b). Because of the inconclusive assessment of its genotoxicity, the FEEDAP Panel was unable to conclude on the safety of α‐damascone [07.134] (EFSA FEEDAP Panel, 2020). In the absence of new data that would allow the FEEDAP Panel to rule out the genotoxicity concern for α‐damascone [07.134], the FEEDAP Panel is unable to conclude on the safety for the user for this compound.
The revised maximum safe levels for the 30 remaining compounds are not expected to further impact on the previous conclusions on user safety.
3.2.4. Safety for the environment
In its previous assessments, the FEEDAP Panel concluded that the use of the majority of the 31 compounds in animal feed at the maximum safe level for the target species is considered safe for the environment.
For three compounds belonging to CG 8, α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224], the predicted environmental concentration for soil (PECsoil) arising from the application rate of 1.5 mg/kg exceeded the threshold of 10 μg/kg. For these compounds, it was not possible to derive a lethal concentration (LC50) for the earthworms using ECOSAR. Therefore, the FEEDAP Panel could not reach a conclusion on the safety of these compounds for the terrestrial compartment (EFSA FEEDAP Panel, 2016c). The FEEDAP Panel was unable to conclude on the safety of α‐damascone [07.134] because of the inconclusive assessment of its genotoxicity (EFSA FEEDAP Panel, 2020).
For a number of compounds, 2‐methyl‐1‐phenylpropan‐2‐ol [02.035] in CG 6, α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224] in CG 8, phenethyl isovalerate [09.466] in CG 15, 4‐(p‐hydroxyphenyl) butan‐2‐one [07.055] in CG 21 and 2‐isopropyl‐4‐methylthiazole [15.026] in CG 29, the FEEDAP Panel identified a potential concern for the use in marine aquaculture (sea cages) at the use levels considered safe for the target species and estimated a safe level of 0.05 mg/kg feed (EFSA FEEDAP Panel, 2012b,f, 2016b,c,d).
To support the safety of use levels in feed higher than those considered safe for the environment in the previous assessments, the applicant provided experimental data, which would allow the FEEDAP Panel to revisit the conclusions on the safety for the environment for the 22 compounds under assessment and made a proposal to extrapolate to the remaining nine compounds.
At the end of the tolerance trials, samples of faeces and urine were collected from animals from the control group and from the group administered with the maximum recommended level (1× MRD). For piglets, faecal samples (two animals per pen, all pens) and urine (one animal per pen, 2 pens per treatment) were collected at day 42. For cattle for fattening, faeces and pen manure samples were collected at day 42 from all animals and urine samples from two pens per treatment. For chickens for fattening, samples of excreta were collected at day 36 (from one animal per pen, all pens). The concentrations of the 22 components of the mixture were determined in all samples.
For each component, the fraction of the dose considered to be active (FA) was calculated as the ratio between the average concentration in manure at 1× MRD (corrected by the concentration in control) and the theoretical concentration of the compounds fed to the animals.
The concentration of the additives in manure from the control group and the group receiving 1 × MRD was calculated from the average concentrations of the additives in faeces and urine sample as follow:
where piglet total manure is 84 kg (45 kg dung and 39 kg urine) and cattle for fattening total manure is 58 kg (40 kg dung and 18 kg urine).13 The FEEDAP Panel notes that the metabolism study submitted does not comply with the provisions of the guidance (EFSA FEEDAP Panel, 2019a,b). Particularly, the volume of excreta produced was not measured and default values (without a range of variability) were used to calculate the concentration in manure.
The concentrations in manure determined in samples taken at the end of the tolerance studies in poultry, pigs and cattle for fattening are summarised in Table 6.
Table 6.
Concentrations in manure of the 22 compounds tested in tolerance trials with ‘TuttiFrutti’ (M2) mixture(a)
| CG | EU register name | FLAVIS no | Use level | Manure levels | Conclusion | ||
|---|---|---|---|---|---|---|---|
| Poultry | Pigs | Cattle | |||||
| mg/kg | % FA | ||||||
| 01 | Dodecanal | 05.011 | 5 | 43% | 1.39% | 0 | Extensively metabolised in pigs and cattle but not in poultry and natural occurrence (> 5 mg/kg) |
| 01 | Ethyl heptanoate | 09.033 | 25 | 1% | 1.8% | 0.5% | Extensively metabolised |
| 01 | Ethyl 2‐methylbutyrate | 09.409 | 25 | 0 | 0.8% | 0 | Extensively metabolised |
| 02 | Isopentyl acetate | 09.024 | 125 | 0.84% | 0 | 0 | Extensively metabolised |
| 02 | 3‐Methylbutyl 3‐methylbutyrate | 09.463 | 25 | 1.02% | 0.2% | 0.14% | Extensively metabolised |
| 03 | Hex‐2‐en‐1‐ol | 02.020 | 5 | 0 | 0.26% | 7.61% | Extensively metabolised |
| 03 | Hex‐2(trans)‐enal | 05.073 | 5 | 113.6% | 0.01 | 1.33% | Extensively metabolised in pigs and cattle but not in poultry and natural occurrence (> 5 mg/kg) |
| 03 | Allyl hexanoate | 09.244 | 5 | 0 | 1.3% | 0 | Extensively metabolised |
| 06 | Linalool | 02.013 | 30 | 0.46% | 0.12% | 0.07% | Natural occurrence and extensively metabolised |
| 06 | 2‐Methyl‐1‐phenylpropan‐2‐ol | 02.035 | 5 | 0.18% | 0.54% | 0.46% | Extensively metabolised |
| 08 | α‐Ionone | 07.007 | 25 | 0.9% | 1.02% | 0.17% | Natural occurrence and extensively metabolised |
| 08 | Nootkatone | 07.089 | 5 | 0 | 2.43% | 0.05% | Natural occurrence and extensively metabolised |
| 08 | β‐Damascone | 07.083 | 5 | 4.2% | 9.06% | 3.73% | |
| 08 | α‐Damascone | 07.134 | 5 | 6.27% | 28.6% | 6.9% | |
| 09 | Pentadecano‐1,15‐lactone | 10.004 | 5 | 0 | 6.71% | 4.78% | Extensively metabolised |
| 15 | 2‐Phenylethan‐1‐ol | 02.019 | 25 | 0.61% | 0.30% | 0.47% | Extensively metabolised |
| 15 | Phenethyl isovalerate | 09.466 | 25 | 1.66% | 0.15% | 0.29% | Extensively metabolised |
| 20 | 8‐Mercapto‐p‐menthan‐3‐one | 12.038 | 0.5 | 0 | 1.12% | 0.46% | Extensively metabolised |
| 21 | 4‐(p‐Hydroxyphenyl) butan‐2‐one | 07.055 | 25 | 0 | 0.52% | 1.02% | Extensively metabolised |
| 26 | 2‐Methoxynaphthalene | 04.074 | 1.2 | 10.4% | 10.8% | 3.61% | Metabolised (90%) |
| 29 | 2‐Isopropyl‐4‐methylthiazole | 15.026 | 1.5 | 1.53% | 0 | 0.72%) | Extensively metabolised |
| 31 | Valencene | 01.017 | 5 | 0 | 3.41% | 2.05% | Extensively metabolised and natural occurrence (> 5 mg/kg) |
The concentrations in manure were calculated from the concentrations determined in faeces and urine samples taken at the end of the tolerance studies in pigs and cattle for fattening and in excreta sample taken at the end of the tolerance study in poultry. The concentrations are expressed as the percentage of fraction of the dose considered to be active (%FA).
The analytical results expressed as %FA indicate that all compounds tested are extensively metabolised in the target species, the fraction in manure being < 5% of the theoretical concentration fed to the animals. The data confirm the hypothesis made by the FEEDAP Panel that compounds belonging to CG 1, 2, 3, 9 and 31 are extensively metabolised in the animals, with the exception of dodecanal [05.011] and hex‐2(trans)‐enal [05.073] in poultry. For these compounds, the applicant provided evidence that they are naturally occurring in plants at concentrations higher than the proposed use level.14 , 15 Extensive metabolism in all species was also demonstrated for compounds belonging to CG 6, 15, 20, 21 and 29, and for α‐ionone [07.007] and nootkatone [07.089] in CG 8. Owing to the structural and metabolic similarities, the conclusions for α‐ionone are extrapolated to α‐irone [07.011] (see Section 3.2.1.6).
For the remaining compounds, β‐damascone [07.083] and α‐damascone [07.134] in CG 8 the %FA is up to 9% and 28.6% in pig manure, respectively. For 2‐methoxynaphthalene [07.074] in CG 26, the %FA is up to 10.8% in pig manure.
For β‐damascone [07.083], the PECsoil arising from the application rate of 1.5 mg/kg exceeded the threshold of 10 μg/kg and in the absence of an LC50 for earthworms, the FEEDAP Panel could not conclude on the safety for the terrestrial compartments. However, when the % fraction in manure of β‐damascone was used to refine the calculations for PECsoil at the application rate of 5 mg/kg, it resulted below the trigger of 10 μg/kg.16 However, according to guidance on the environmental risk assessment (EFSA, 2008), the refined PECsoil in Phase II needs the comparison with a predicted no effect concentration (PNEC) estimate for the terrestrial compartment. Therefore, in the absence of ecotoxicity data, the FEEDAP Panel cannot conclude on the safety of β‐damascone [07.083] for the terrestrial compartments. The same conclusion also applies to the non‐tested compound (E)‐β‐damascone [07.224], whereas for β‐damascenone, the applicant provided evidence that it is naturally occurring in plant at concentrations higher than the proposed use level.
For α‐damascone [07.134], because of the inconclusive assessment of its genotoxicity, the FEEDAP Panel did not perform an assessment of the safety for the environment. In the absence of data, the FEEDAP Panel cannot conclude on the safety of α‐damascone for the environment.
For 2‐methoxynaphthalene [04.074] and other compounds belonging to CG 26, the FEEDAP Panel concluded that ‘at a dose of 1 mg/kg these compounds are not expected to pose a risk for the environment. Their environmental consequences when used at a dose of 5 mg/kg complete feed are less certain and may result in PNECs being exceeded in both water and soil compartments’ (EFSA FEEDAP Panel, 2012c). In the absence of data to support the safety of the proposed use level of 1.2 mg/kg, the extrapolation of the conclusions of the former assessment to a 20% higher concentration in feed is uncertain.17 However, the FEEDAP Panel notes the probability of effects would be very low at 1.2 mg/kg.
For those compounds for which the FEEDAP Panel has identified a potential concern for the marine environment (sea cages), namely 2‐methyl‐1‐phenylpropan‐2‐ol [02.035] in CG 6, α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224] in CG 8, phenethyl isovalerate [09.466] in CG 15, 4‐(p‐hydroxyphenyl) butan‐2‐one [07.055] in CG 21, and 2‐isopropyl‐4‐methylthaizole [15.026] in CG 29, the applicant proposed to limit the concentration in fish feed used in marine aquaculture to 0.05 mg/kg.
3.2.4.1. Conclusions on safety for the environment
In the absence of data, the FEEDAP Panel cannot conclude on the safety of α‐damascone [07.134] for the environment. The concentrations considered safe for the target species are unlikely to have detrimental effects on the environment for all the compounds except for β‐damascone [07.083] and (E)‐β‐damascone [07.224], for which in the absence of ecotoxicity data, the FEEDAP Panel cannot conclude on the safety for the terrestrial compartment.
For the marine environment, the safe use level for 2‐methyl‐1‐phenylpropan‐2‐ol [02.035], α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224], phenethyl isovalerate [09.466], 4‐(p‐hydroxyphenyl) butan‐2‐one [07.055] and 2‐isopropyl‐4‐methylthiazole [15.026] is confirmed to be 0.05 mg/kg.
4. Conclusions
The conclusions of the FEEDAP Panel on the maximum safe concentration of the 31 compounds in complete feed for all animal species are summarised in the following table:
| CG | Product (EU register name) | FLAVIS no | All animal species (mg/kg complete feed) |
|---|---|---|---|
| 01 | Dodecanal | 05.011 | 5 |
| Ethyl heptanoate | 09.093 | 31.8 | |
| Ethyl 2‐methylbutyrate | 09.409 | 25 | |
| 02 | 2‐Methylpropionic acid | 08.006 | 25 |
| Isopentyl acetate | 09.024 | 125 | |
| 3‐Methylbutyl butyrate | 09.055 | 25 | |
| 2‐Methylbutyl acetate | 09.286 | 25 | |
| 3‐Methylbutyl 3‐methylbutyrate | 09.463 | 25 | |
| 03 | Hex‐2‐en‐1‐ol | 02.020 | 5 |
| Hex‐2(trans)‐enal | 05.073 | 5 | |
| Allyl hexanoate | 09.244 | 5 | |
| Allyl heptanoate | 09.097 | 5 | |
| 06 | Linalool | 02.013 | 30 |
| 2‐Methyl‐1‐phenylpropan‐2‐ol | 02.035 | 5 | |
| 08 | α‐Ionone | 07.007 | 25 |
| β‐Ionone | 07.008 | 1–5* | |
| α‐Irone | 07.011 | 5 | |
| β‐Damascone | 07.083 | 5 | |
| Nootkatone | 07.089 | 5 | |
| β‐Damascenone | 07.108 | 1.0–1.5** | |
| (E)‐β‐Damascone | 07.224 | 5 | |
| α‐Damascone | 07.134 | 5*** | |
| 09 | Pentadecano‐1,15‐lactone | 10.004 | 10 |
| 15 | 2‐Phenylethan‐1‐ol | 02.019 | 25 |
| Phenethyl isovalerate | 09.466 | 30 | |
| 20 | 8‐Mercapto‐p‐menthan‐3‐one | 12.038 | 0.5 |
| p‐Menth‐1‐ene‐8‐thiol | 12.085 | 0.5 | |
| 21 | 4‐(p‐Hydroxyphenyl) butan‐2‐one | 07.055 | 25 |
| 26 | 2‐Methoxynaphthalene | 04.074 | 1.2 |
| 29 | 2‐Isopropyl‐4‐methylthiazole | 15.026 | 1.5 |
| 31 | Valencene | 01.017 | 5 |
Safe at 5 mg/kg feed for salmonids, veal calves and dogs, and at 1 mg/kg feed for the remaining species.
Safe at 1.5 mg/kg for cattle for fattening, salmonids and non‐food producing animals, and 1.0 mg/kg for pigs and poultry.
Safe at 5 mg/kg complete feed for chickens for fattening, piglets and cattle for fattening.
In the absence of data that would allow the FEEDAP Panel to rule out the genotoxicity concern, the FEEDAP Panel cannot extend the conclusions for α‐damascone [07.134] to all animal species and cannot conclude on the safety of this compound for the consumer, the user and the environment.
No safety concern would arise for the consumer from the use of the remaining 30 compounds up to the highest levels considered safe for target animals.
The revised maximum safe levels for the 30 remaining compounds are not expected to further impact on the previous conclusions reached on user safety.
The concentrations considered safe for the target species are unlikely to have detrimental effects on the environment for all the compounds except β‐damascone [07.083] and (E)‐β‐damascone [07.224], for which in the absence ecotoxicity data, the FEEDAP Panel cannot conclude on the safety for the terrestrial compartment. For the marine environment, the safe use level for 2‐methyl‐1‐phenylpropan‐2‐ol [02.035], α‐irone [07.011], β‐damascone [07.083] and (E)‐β‐damascone [07.224], phenethyl isovalerate [09.466], 4‐(p‐hydroxyphenyl) butan‐2‐one [07.055] and 2‐isopropyl‐4‐methylthiazole [15.026] is confirmed to be 0.05 mg/kg.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 06/05/2015 | Info session held in Barcelona, where a general discussion took place on how to follow up a series of inconclusive opinions on the safety of the proposed use levels of certain chemically defined flavourings |
| 11/05/2016 | Technical hearing during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products, held in Milan (FEEDAP working group on guidance update) |
| 02/12/2019 | Dossier received by EFSA. Safety of 31 flavouring compounds belonging to different chemically defined groups for all animal species. Submitted by FEFANA asbl |
| 10/01/2020 | Reception mandate from the European Commission |
| 27/02/2020 | Application validated by EFSA – Start of the scientific assessment |
| 29/06/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety for the target species, safety for the environment |
| 26/08/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 18/11/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ALP
alkaline phosphatase
- ANOVA
Analysis of variance
- CG
chemical group
- DM
dry matter
- ECOSAR
Component program of EPI Suite™
- EURL
European Union Reference Laboratory
- FA
fraction of the dose considered to be active
- FGE
food group evaluation
- FLAVIS
The EU Flavour Information System
- FL‐no
FLAVIS number
- GC‐MS
gas chromatography‐mass spectrometry
- GLM
Generalised linear models
- MRD
maximum recommended dose
- NOAEL
no observed adverse effect level
- PECsoil
Predicted environmental concentration for soil
- PNEC
Predicted no effect concentrations
- SAS
Statistical Analysis System
- TTC
threshold of toxicological concern
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Durjava MF, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Dierick N, Martelli G, Westendorf J, Anguita M, Galobart J and Manini P, 2020. Scientific Opinion on the safety of 31 flavouring compounds belonging to different chemical groups when used as feed additives for all animal species. EFSA Journal 2020;18(12):6338, 22 pp. 10.2903/j.efsa.2020.6338
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00175
Panel members: Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The Panel wishes to acknowledge the contribution of Antonio Finizio, Andreas Focks, Matteo Lorenzo Innocenti, Fabiola Pizzo, Jordi Tarrés‐Call and Ivana Teodorovic to this opinion.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 18 November 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96 of the European Parliament and of the Council. OJ L 180, 19.7.2000, p. 8.
FEED dossier reference: FAD‐2019‐0092.
FEED dossiers’ reference: FAD‐2010‐0015, FAD‐2010‐0013, FAD‐2010‐0416, FAD‐2010‐0025, FAD‐2010‐0125, FAD‐2010‐0414, FAD‐2010‐0097, FAD‐2010‐0027, FAD‐2010‐0409, FAD‐2010‐0075, FAD‐2010‐0054, FAD‐2010‐0410, FAD‐2010‐0411.
The full reports are available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0015.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0013.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0124.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0025.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0125.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0097.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0027.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0043.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0075.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0054.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0116.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0022.pdf.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Total count for erythrocytes, packed cell volume, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, total and differential counts for leukocytes, platelet counts.
Sodium, potassium, chloride, calcium, phosphate, magnesium, total protein, albumin, globulin, glucose, uric acid, cholesterol, creatinine, bilirubin, acute phase protein, amylase, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), gamma‐glutamyl transferase (GGT), alkaline phosphatase (ALP), and creatine kinase.
Total count for erythrocytes, packed cell volume, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, total and differential counts for leucocytes, platelet counts.
Sodium, potassium, chloride, calcium, phosphate, magnesium, total protein, albumin, globulin, glucose, uric acid, cholesterol, creatinine, bilirubin, acute phase protein, amylase, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), gamma‐glutamyltransferase (GGT), alkaline phosphatase (ALP), creatine kinase, prothrombin time and fibrinogen.
Total count for erythrocytes, packed cell volume, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, total and differential counts for leucocytes, platelet counts.
Alkaline phosphatase, amylase, gamma‐glutamyl transferase, alanin aminotransferase, aspartate aminotransferase, lactate dehydrogenase, creatine kinase, calcium, phosphate, magnesium, potassium, sodium, chloride, cholesterol, lactic acid, albumin, total protein, urea, creatinine.
Technical dossier/Supplementary information August 2020.
Technical dossier FAD‐2010‐0015/Supplementary information May 2011/Annex TNO_2010_FL‐05.011.
Technical dossier/Report and Annexes/EFSA_TT_M2_Annex_ERA_CG03/Annex_2_TNO_2017_CG03_05_073.
Technical dossier/Report and Annexes/EFSA_TT_M2_Annex_ERA_CG08.
Technical dossier/Report and Annexes/EFSA_TT_M2_Annex_ERA_CG26.
References
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Scientific Opinion on the safety and efficacy of phenyl ethyl alcohols, phenylacetic acids, related esters, phenoxyacetic acids and related esters (chemical group 15) when used as flavourings for all animal species. EFSA Journal 2012;10(3):2625, 16 pp. 10.2903/j.efsa.2012.2625 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Scientific Opinion on the safety and efficacy of aromatic ethers including anisole derivatives (chemical group 26) when used as feed additives for all animal species. EFSA Journal 2012;10(5):2678, 19 pp. 10.2903/j.efsa.2012.2678 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Scientific Opinion on the safety and efficacy of primary aliphatic saturated or unsaturated alcohols/aldehydes/acids/acetals/esters with a second primary, secondary or tertiary oxygenated functional group including aliphatic lactones (chemical group 9) when used as flavourings for all animal species. EFSA Journal 2012;10(10):2928, 24 pp. 10.2903/j.efsa.2012.2928 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012e. Scientific Opinion on the safety and efficacy of branched‐chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing branched‐chain alcohols and acetals containing branched‐chain aldehydes (chemical group 2) when used as flavourings for all animal species. EFSA Journal 2012;10(10):2927, 26 pp. 10.2903/j.efsa.2012.2927 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012f. Scientific Opinion on the safety and efficacy of aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers (chemical group 6) when used as flavourings for all animal species. EFSA Journal 2012;10(11):2966, 25 pp. 10.2903/j.efsa.2012.2966 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013. Scientific Opinion on the safety and efficacy of straight‐chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes (chemical group 01) when used as flavourings for all animal species. EFSA Journal 2013;11(4):3169, 35 pp. 10.2903/j.efsa.2013.3169 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical Group 31) when used as flavourings for all animal species and categories. EFSA Journal 2016;14(1):4339, 17 pp. 10.2903/j.efsa.2016.4339 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016b. Scientific opinion on the safety and efficacy of thiazoles, thiophene and thiazoline belonging to chemical group 29 when used as flavourings for all animal species. EFSA Journal 2016;14(6):4441, 16 pp. 10.2903/j.efsa.2016.4441 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016c. Scientific opinion on the safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species. EFSA Journal 2016;14(6):4475, 26 pp. 10.2903/j.efsa.2016.4475 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016d. Scientific opinion on the safety and efficacy of aromatic ketones, secondary alcohols and related esters belonging to chemical group 21 when used as flavourings for all animal species. EFSA Journal 2016;14(8):4557, 17 pp. 10.2903/j.efsa.2016.4557 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Westendorf J, Gregoretti L, Manini P and Dusemund B, 2019a. Scientific Opinion on the safety and efficacy of 8‐mercapto‐p‐menthan‐3‐one and p‐menth‐1‐ene‐8‐ thiol belonging to chemical group 20 when used as flavourings for all animal species. EFSA Journal 2019;17(1):5530, 12 pp. 10.2903/j.efsa.2019.5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Westendorf J, Gregoretti L, Manini P and Dusemund B, 2019b. Scientific Opinion on the safety and efficacy of 26 compounds belonging to chemical group 3 (α,β‐unsaturated straight‐chain and branched‐chain aliphatic primary alcohols, aldehydes, acids and esters) when used as flavourings for all animal species and categories. EFSA Journal 2019;17(3):5654, 16 pp. 10.2903/j.efsa.2019.5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Westendorf J, Gregoretti L, Manini P and Dusemund B, 2020. Scientific Opinion on the safety and efficacy of oct‐1‐en‐3‐ol, pent‐1‐en‐3‐ol, oct‐1‐en‐3‐one, oct‐1‐en‐ 3‐yl acetate, isopulegol and 5‐methylhept‐2‐en‐4‐one, belonging to chemical group 5 and of isopulegone and alpha‐damascone belonging to chemical group 8 when used as flavourings for all animal species. EFSA Journal 2020;18(2):6002, 16 pp. 10.2903/j.efsa.2020.6002 [DOI] [PMC free article] [PubMed] [Google Scholar]


