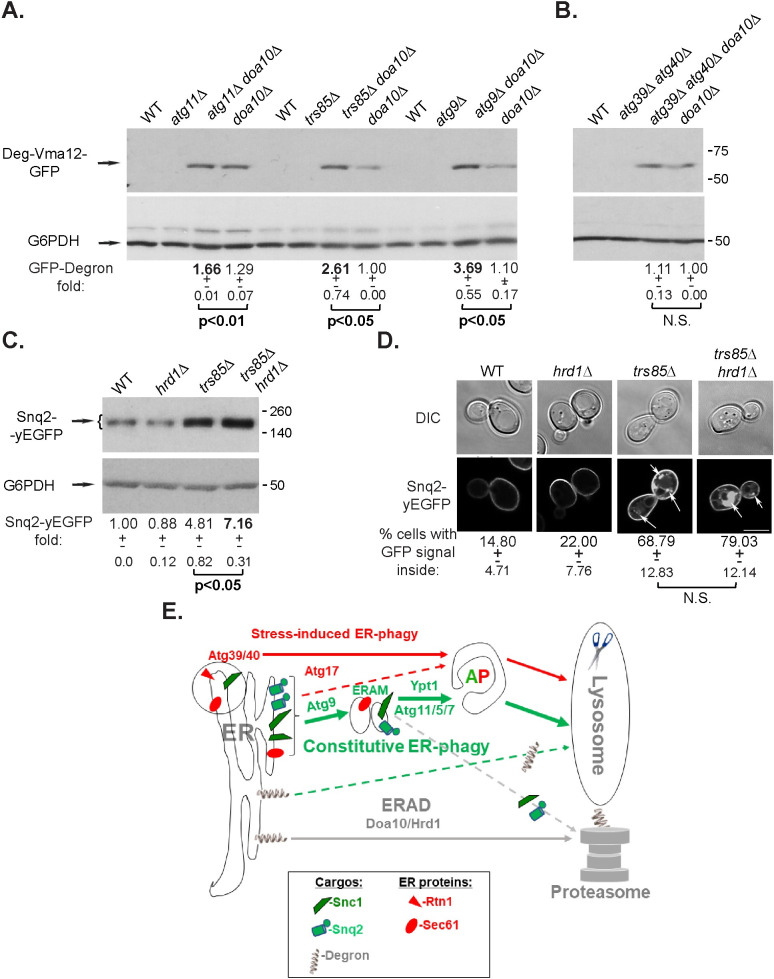
Fig 7. Partial overlap between constitutive ER-phagy and ERAD.
A-B. Effect of constitutive ER-phagy mediators, but not of Atg39 and Atg40, on clearance of an ERAD-C cargo. A. Deletion of ATG11, TRS85 and ATG9 alone do not result in accumulation of an ERAD-C substrate, but they do increase the doa10Δ ERAD-C phenotype. WT and indicated mutant cells expressing Deg1-Vma12-GFP (integrated into the TRP1 locus [29]) were grown in YPD. The level of Deg1-Vma12-GFP was determined by immuno-blot analysis using anti-GFP antibodies. Shown from top to bottom: strain, GFP blot, G6PDH blot (loading control), fold GFP-degron over the WT level, +/- STD, and p-value. Combining atg11Δ, trs85Δ or atg9Δ, with doa1Δ results in a significant increase of the GFP-Degron level. B. Deletion of ATG39 ATG40 alone or in combination with doa10Δ does not result in accumulation of an ERAD-C substrate or an increase in the ERAD phenotype of doa10Δ. Experiments were done as in panel A. C-D. While deletion of HRD1 alone does not result in Snq2-yEGFP accumulation, it does increase the defect of trs85Δ. WT and indicated mutant cells overexpressing Snq2-yEGFP were grown in normal growth medium (SD+N). The level of Snq2-yEGFP in cell lysates was determined by immuno-blot analysis using anti-GFP antibodies (C); intracellular accumulation of Snq2-yEGFP was determined by live-cell fluorescence microscopy (D). Results are presented as in Fig 2. A ~50% increase in the level of Snq2-yEGFP is observed when hrd1Δ is combined with trs85Δ. Results in this figure represent 3 independent experiments. E. Model showing the three ER quality control pathways discussed here: First, constitutive ER-phagy (green) shuttles overexpressed membrane proteins, e.g., Snc1 and Sec61, to the lysosome for degradation via autophagy. It requires, among other Atgs, Atg9, Ypt1, Atg11, Atg5 and Atg7. While Atg9 is required for sequestration of constitutive ER-phagy cargo into ER-associated membranes (ERAM), Ypt1 (and its Trs85-containing GEF), Atg11, Atg5 and Atg7 function in a later step of autophagosome (AP) assembly [7]. Second, Doa10- and Hrd1-mediated ERAD-C/M shuttle specific cargos, e.g., Deg1-Vma12-GFP, for degradation by the proteasome (gray). Both these processes operate during normal growth and depletion of each one does not affect the function of the other. However, when either constitutive ER-phagy or ERAD are compromised, the other process can clear some of the cargo (dashed lines). Third, nutritional stress-induced ER-phagy (red) shuttles ER fragments containing ER proteins, e.g., Rtn1, including constitutive ER-phagy cargo, e.g., Snc1 and Sec61, to the lysosome for degradation via APs. This process requires Atg17 and the Atg8-receptors Atg39 and Atg40. While these two receptors are not required for and do not affect neither constitutive ER-phagy nor ERAD, Atg17-dependent autophagy can clear constitutive ER-phagy cargo during normal growth in atg11Δ mutant cells (dashed red line).