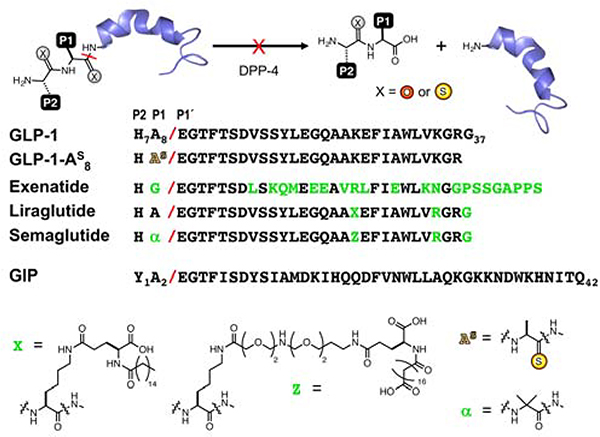
Figure 1.
Thioamides Prevent Peptide Inactivation by Proteolysis. Native peptides are inactivated by DPP-4 cleavage at the scissile bond, indicated with a red slash (P2, P1 and P1’ positions are numbered relative to the scissile bond, by convention). Sequences of GLP-1, GLP-1 analogs, and GIP are shown. GLP-1 analogs exenatide, liraglutide, and semaglutide are stabilized by extensive mutation, sidechain fatty acid modification (X or Z), or a combination of fatty acid modification and aminoisobutyric acid (α) incorporation, respectively. Other GLP-1 stabilization strategies are described in the text. Thioamide substitution is indicated by an “S” superscript.