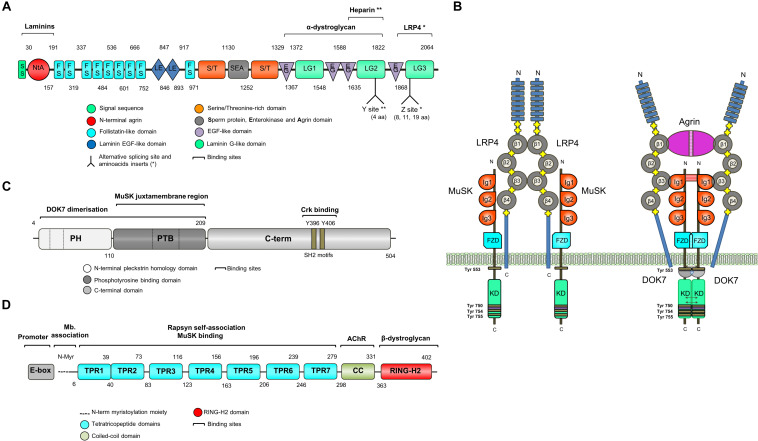
FIGURE 3.
Schematic representation of key NMJ proteins. (A) Agrin (RefSeq NP_001292204.1) is a large proteoglycan (>200 KDa) with multiple domains that binds to laminins through the N-terminal domain, and to LRP4 and α-dystroglycan via C-terminus. Two of the three laminin G-like domains are required for binding to α-dystroglycan. Agrin mRNA undergoes cell-specific alternative splicing at different sites (Ferns et al., 1993). When agrin is produced by motor neurons, the Z site contain amino acid inserts specifically required for MuSK activation. (B) The binding of agrin to the N-terminal region of LRP4 induces a conformational change (active state) promoting the binding between LRP4 and the first IgG-like domain of MuSK and the formation of a tetrameric complex (Zhang et al., 2011). This results in MuSK activation via dimerization and trans-autophosphorylation of tyrosine residues within the cytoplasmic region (Schlessinger, 2000). The increase in the catalytic activity creates active binding sites for other proteins such as DOK7, leading to in full kinase activation and amplification of the signal downstream. The composition of LRP4 includes 8 Low density lipoprotein Class A (LDLa) domains (blue) at the N terminus, followed by 4 YWTD ß-propeller domains (gray) bounded by epidermal growth factor-like modules (yellow) and a short C-terminal domain (Springer, 1998). LRP4 self-associates and interacts with MuSK in the absence of agrin (inactive state), but is not capable of activating MuSK (Kim et al., 2008). (C) DOK7 (RefSeq NM_173660.4) is composed of an N-terminal pleckstrin homology (PH) domain, a phosphotyrosine-binding (PTB) domain and a C-terminus. Dashes are used to mark the different exons. (D) Rapsyn (RefSeq NP_005046.2) is composed of a N-terminal myristoylation moiety (N-Myr) necessary for submembrane localization; seven tetratricopeptide (TRP) domains responsible for rapsyn self-association and MuSK binding (Ramarao et al., 2001; Lee et al., 2008); a coiled-coil domain that binds to the cytoplasmic loops of AChRs (Lee et al., 2008), and a RING-H2 domain that interacts with the dystroglycan complex and links with the cytoskeleton (Apel et al., 1995).