Figure 1.
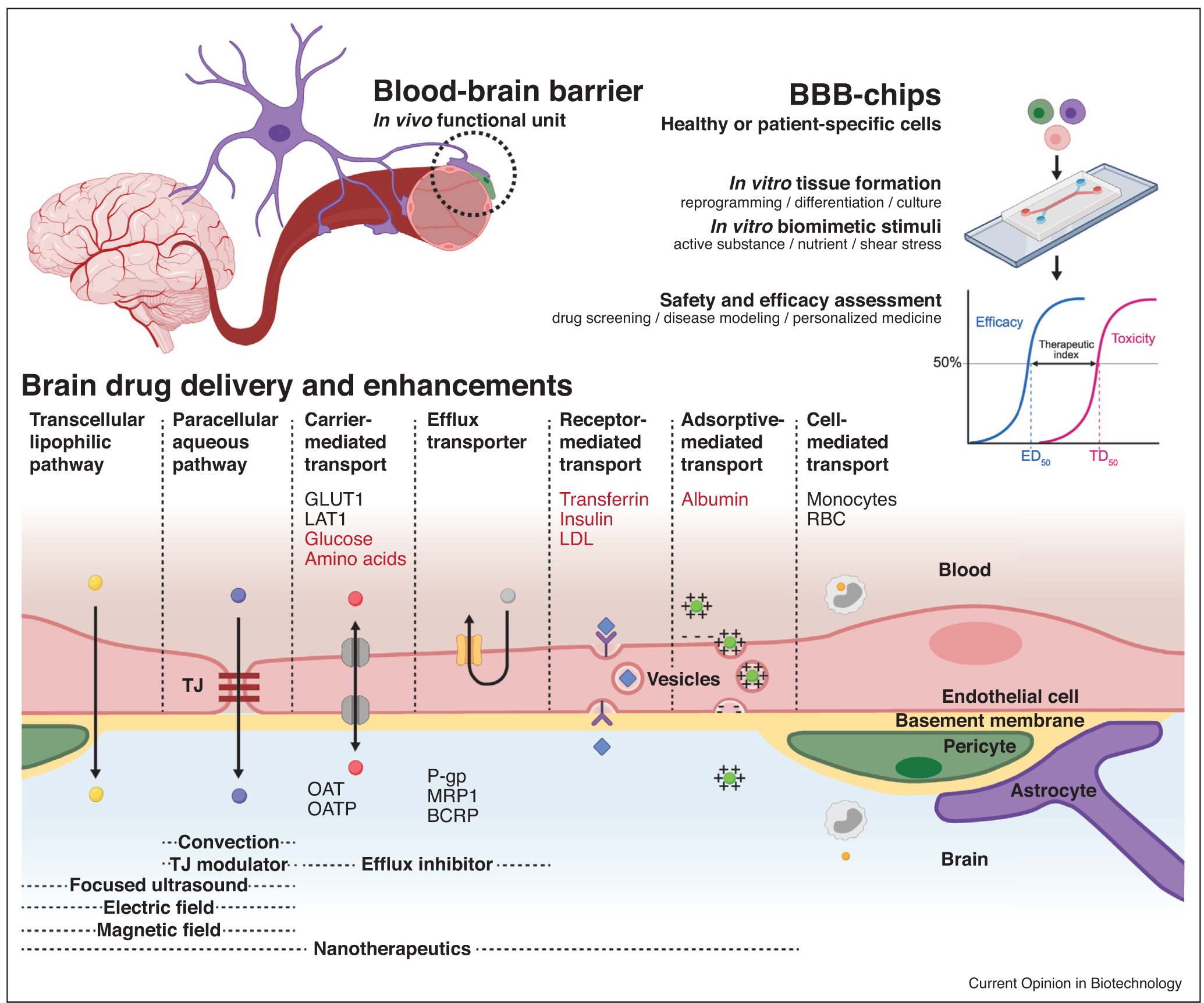
Rationale for development of BBB-chips. BBB-chips can culture cells and apply controlled stimuli that are biomimetic of the human BBB’s functional unit, allowing preclinical assessment of drug candidate safety and efficacy. Playing a critical role in the BBB, TJs are composed of membrane proteins anchored to and strengthened by networks like zonula occludens-1 (ZO-1), forming a tremendous barrier. Still, researchers have been able to deliver drugs to the brain using transcellular lipophilic pathway, paracellular aqueous pathway, and transports mediated by solute carriers (SLC), receptors, adsorptives, and cells [7,8]. Biological molecules that can cross the BBB are shown in red and are widely studied as surface functionalization motifs for brain targeting nanotherapeutics. Main biological components involved in the brain penetration–glucose transporter 1 (GLUT1), large neutral amino acid transporter (LAT1), monocytes, and red blood cells (RBCs)–are indicated on the blood side. Inversely, efflux transporters of both the SLC and ATP-binding cassette (ABC) superfamilies are listed on the brain side of the illustration. Organic anion transporters (OATs/SLC22A), organic anion transporting polypeptides (OATPs/SLCO), P-glycoprotein (P-gp/ABCB1), breast cancer resistance protein (BCRP/ABCG2), and multidrug resistance protein 1 (MRP1/ABCC1) are some of these efflux transporters that can be inhibited to facilitate brain drug delivery. Further biochemical and physical enhancements can be achieved using TJ modulators, convection, focused ultrasound, electrical field, and magnetic field. Growing numbers of BBB-chip studies involve these pathways and enhancements to assess targeted drug delivery to the brain. Partially created with BioRender.com.
