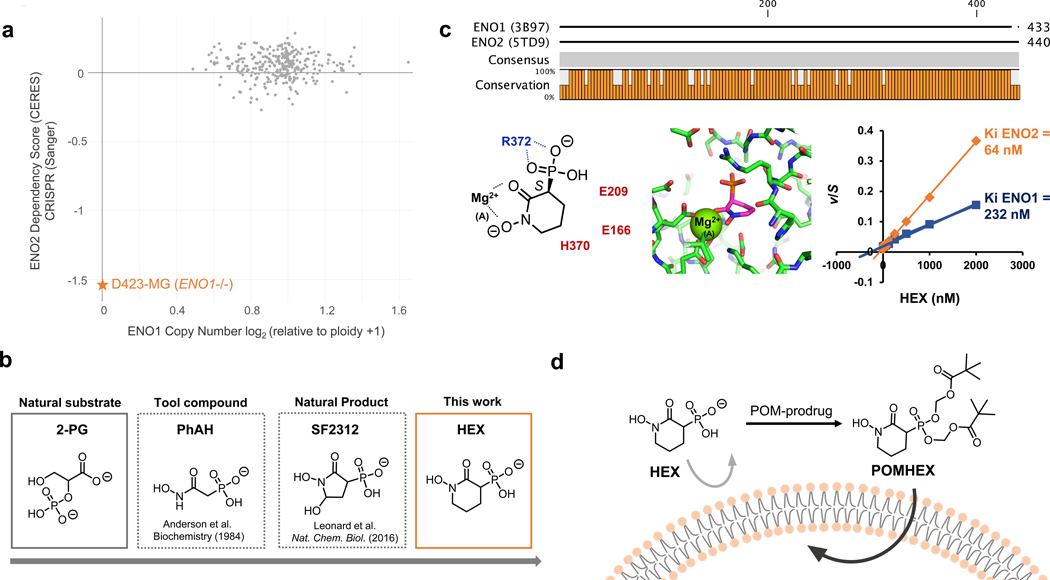
Figure 1. HEX is a substrate-competitive inhibitor of Enolase with specificity for ENO2.
a. Cells with ENO1 homozygous deletions (e.g. D423) are highly dependent on ENO2 to perform glycolysis, as indicated by the ENO2 CERES score. Plot adapted from the Cancer Dependency Map. b. Timeline on the development of ENO2-specific Enolase inhibitors. c. HEX displays ~4-fold specificity for ENO2 over ENO1 despite their structural similarities. Co-crystal structure of HEX and ENO2 (PDB: 5IDZ) indicates that the carbonyl and hydroxamate are crucial for chelating to the active site Mg2+ while the anionic phosphonate forms a salt bridge with R372. HEX shows competitive Michealis-Menten kinetics; plot of apparent Km/Vmax (from Supplementary Figure S1) as a function of HEX concentration (x-axis) for ENO1 and ENO2. d. HEX was modified with POM pro-drug groups to enhance its cell- and blood-brain-barrier permeability.