Figure 3. Dimerization and conserved surfaces of the HsMiro nGTPase.
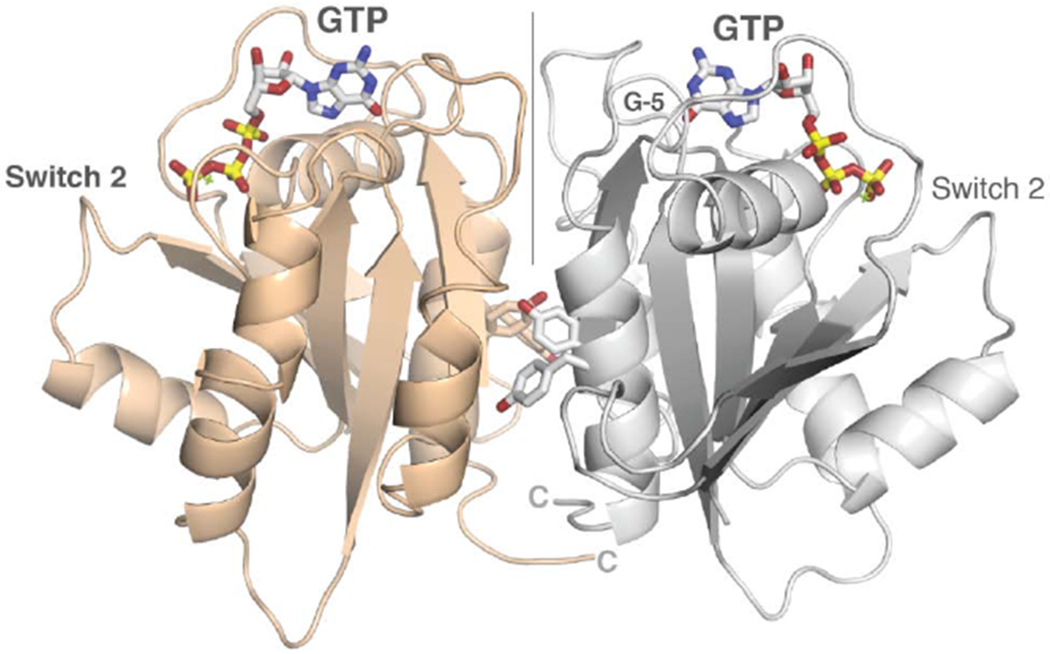
(A) Dimerization of the nGTPase domain in the crystal. The protomers associate across a non-crystallographic two-fold axis. At the center of the interface is a cluster of paired tyrosine sidechains of the ‘SELFYY’ motif. The positions of the GTP molecules are indicated. Note that the interface is distal to the Switch 1 and Switch 2 regions, and therefore is unlikely to be responsive to a specific nucleotide binding state. The G-5 motif in the foreground at right is indicated.
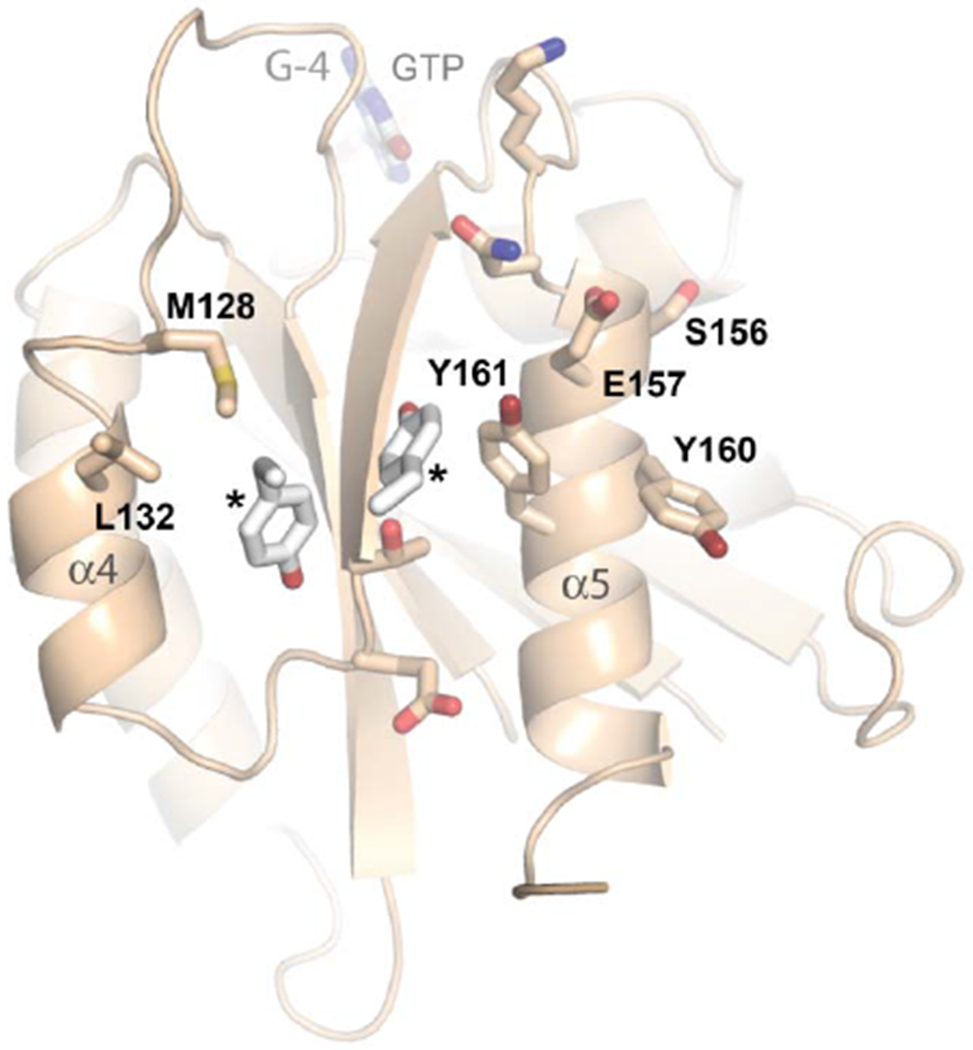
(B) Details of the SELFYY motif interface. One monomer of the dimer is shown rotated 90° relative to (A), with residues substantially buried at the interface shown as sticks. The hydrophobic residues of the interface (M128, L130, Y160, Y161) are labeled, with the positions of the sidechains of the tyrosines extending across the dimer interface (‘*’) shown to highlight their intercalation into a pocket formed by sidechains extending from the α4 and α5 helices. The G-4 loop and GTP are indicated. S156 has been reported to be phosphorylated by PINK1 kinase [70].
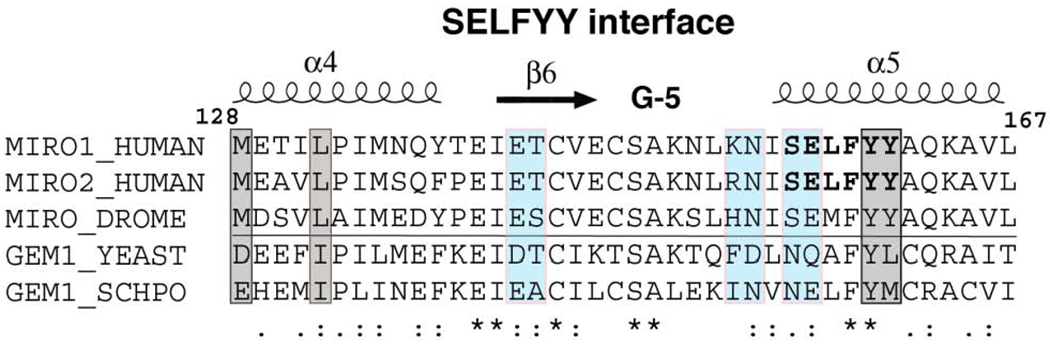
(C) Sequence conservation in the SELFYY interface of the Miro nGTPases. The surface residues that are substantially buried upon formation of the interface are highlighted – hydrophobics in grey, polar residues in teal. The associated secondary structure is indicated above the alignment, sequence conservation indicated below it. (For a more extensive alignment over 36 Miro/Gem1p sequences, see Suppl. Fig. 3.)
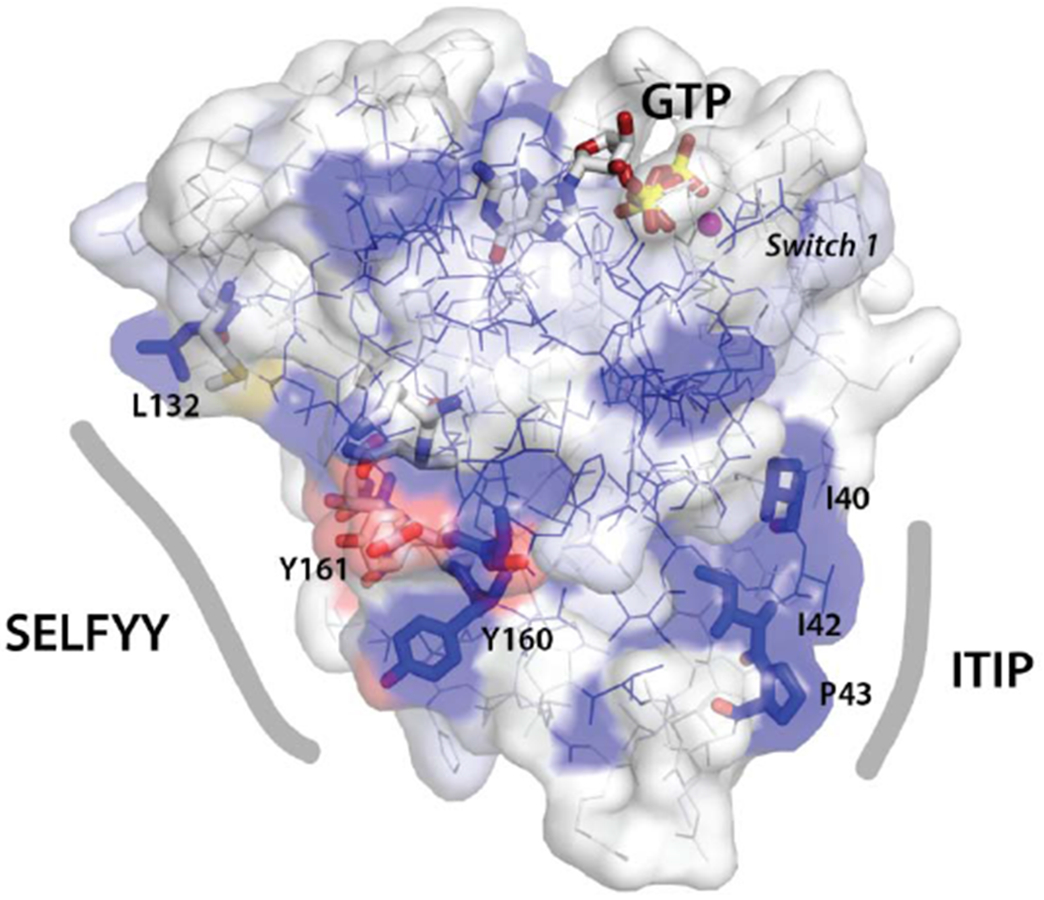
(D) Surface representation of the nGTPase domain. Sequence conservation is mapped as surface color from white (not conserved) to blue (highly conserved). The sidechains of the SELFYY interface and the conserved ‘ITIP’ hydrophobic surface are shown as sticks, and the SW1 region is indicated. The GTP binding site is at top, with GTP shown as sticks. At left, the ‘SELFYY’ surface extends across a broad face of the domain (L132 – Y160); at right the ‘ITIP’ hydrophobic surface (I40 – P43) follows switch 1 (‘SW1’).
