Table 1.
In vitro cytotoxic activity of analogs with the mono-substitution at the “head” moiety.
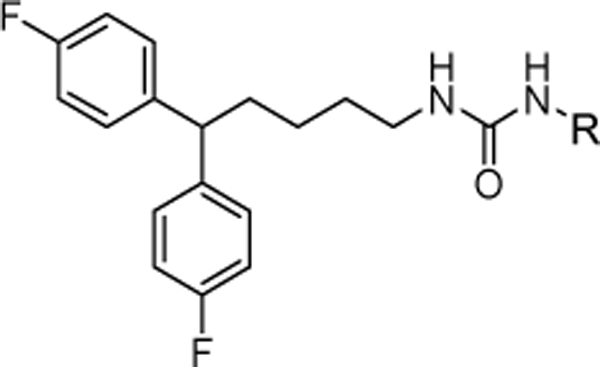 | |||||
|---|---|---|---|---|---|
| R | MDA-MB-231 aIC50 (μM) | R | MDA-MB-231 aIC50 (μM) | ||
| 4a | 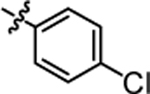 |
7.68 ± 0.21 | 4g | 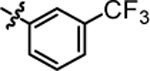 |
3.38 ± 0.08 |
| 4b | 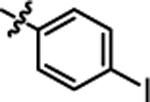 |
11.79 ± 0.40 | 4h | 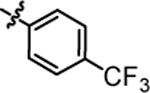 |
2.78 ± 0.09 |
| 4c | 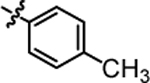 |
>20.00 | 4i | 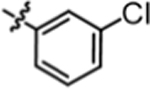 |
5.07 ± 0.33 |
| 4d | 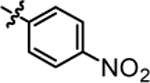 |
6.47 ± 0.84 | 4j | 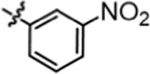 |
3.79 ± 0.22 |
| 4e | 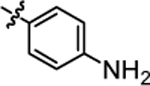 |
>20.0 | 4k | 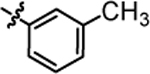 |
>20.0 |
| 4f | >20.0 | ||||
Data are expressed as the mean ± SEM of three independent experiments, each performed in a quartet.
