Table 2.
In vitro cytotoxic activity of analogs with the di-substitution at the “head” moiety.
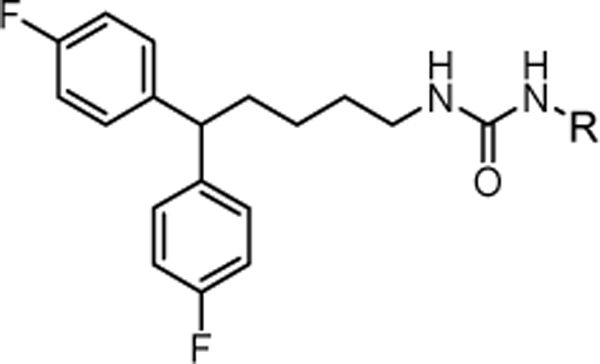 | |||||
|---|---|---|---|---|---|
| R | MDA-MB-231 aIC50 (μM) | R | MDA-MB-231 aIC50 (μM) | ||
| 4l | 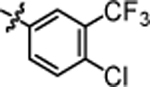 |
3.15 ± 0.11 | 4r | 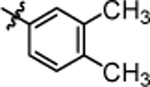 |
>20.0 |
| 4m | 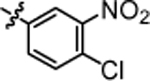 |
4.06 ± 0.74 | 4s | 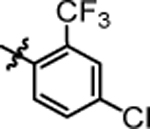 |
>20.0 |
| 4n | 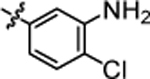 |
9.24 ± 0.29 | 4t | 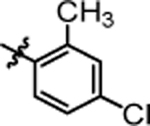 |
>20.0 |
| 4o | 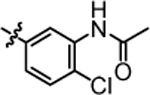 |
9.12 ± 0.43 | 4u | 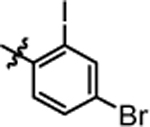 |
>20.0 |
| 4p | 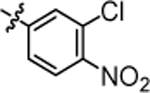 |
3.47 ± 0.14 | 4v | 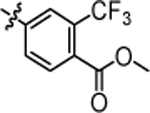 |
4.43 ± 0.23 |
| 4q | 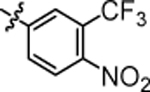 |
4.55 ± 0.12 | 4x | 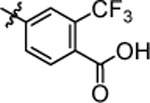 |
>20.0 |
Data are expressed as the mean ± SEM of three independent experiments, each performed in a quartet.
