Table 3.
In vitro cytotoxic activity of analogs with the modifications at the “tail” di-phenyl moiety.
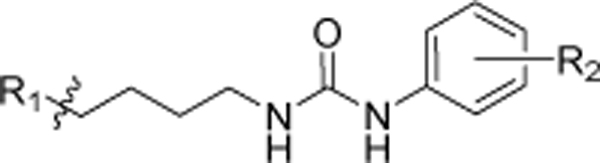 | |||
|---|---|---|---|
| R1 | R2 | MDA-MB-231 aIC50 (μM) | |
| 4l | 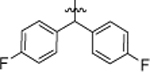 |
4-Cl, 3-CF3 | 3.15 ± 0.11 |
| 8a | 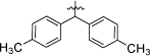 |
4-Cl, 3-CF3 | 2.51 ± 0.09 |
| 8b | 4-Cl | 17.93 ± 0.37 | |
| 8c | 4-Cl, 3-CF3 | 3.30 ± 0.32 | |
| 8d | 4-Cl, 3-NO2 | 3.99 ± 0.44 | |
| 8e | 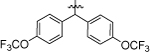 |
4-Cl, 3-CF3 | 1.69 ± 0.11 |
| 8f | 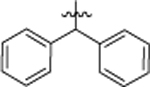 |
4-Cl, 3-CF3 | 5.72 ± 0.26 |
| 8g | 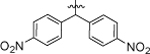 |
4-Cl, 3-CF3 | 1.23 ± 0.20 |
| 8h | 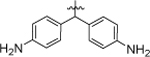 |
4-Cl, 3-CF3 | 12.20 ± 0.59 |
| 8i | 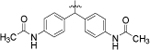 |
4-Cl, 3-CF3 | >20.0 |
| 9a | 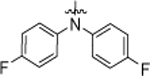 |
4-Cl | >20.0 |
| 9b | 4-I | > 20.0 | |
| 9c | 4-Cl, 3-CF3 | 5.04 ± 0.14 | |
| 9d | 4-CH3, 3-CH3 | >20.0 | |
| 9e | 4-Br, 2-I | > 20.0 | |
| 10 | 4-Cl, 3-CF3 | 5.44 ± 0.59 | |
Data are expressed as the mean ± SEM of three independent experiments, each performed in a quartet.
