Abstract
Heterotopic ossification is often associated with trauma and surgery, and infrequently reported with immobilization due to critical illness. We present 2 patients who developed heterotopic ossification following severe COVID-19 infection. Both patients were middle-aged females who were hospitalized for one month or greater due to COVID-19 requiring mechanical ventilation. Both developed shoulder pain and/or stiffness a few months after discharge, with imaging studies clearly illustrating development of heterotopic ossification around the shoulders. The etiology is unclear, with immobilization and hypoxia being the primary considerations. Physical examination and radiography are essential to diagnosis. Awareness of this complication and early diagnosis may help minimize functional impairment.
Keywords: SARS-CoV-2, COVID-19, Heterotopic ossification, Myositis ossificans
Abbreviations: HO, heterotopic ossification; RT-PCR, reverse transcription polymerase chain reaction; FiO2, fraction of inspired oxygen; ARDS, acute respiratory distress syndrome; GFR, glomerular filtration rate; NSAIDs, nonsteroidal anti-inflammatory drugs
Background
Heterotopic ossification (HO) is the deposition of mature or immature bone within soft tissue, often in a periarticular location [1]. It has been associated with musculoskeletal trauma, surgery, burns, neurologic injury, immobilization, and congenital and metabolic disorders. It has rarely been described as a complication of critical illness. Recently, a case series was published demonstrating HO around the shoulder and hips in four patients with history of COVID-19 requiring mechanical ventilation [2]. We present 2 patients with HO around the shoulders after prolonged hospitalization for COVID-19.
Case presentations
Patient 1
A 51-year-old female with past medical history of hypertension and type 2 diabetes presented with shortness of breath and cough. She was diagnosed with COVID-19 by molecular RT-PCR test from nasal swab. Due to increased work of breathing and worsening hypoxia, she was intubated with high FiO2 requirements and prolonged ICU stay. Treatment included a single dose of tocilizumab (an IL-6 receptor inhibitor), single administration of COVID-19 convalescent plasma, 5-day course of methylprednisolone, and statins (for anti-inflammatory effect). Supportive care included prone positioning. Hospital course was complicated by septic shock, catheter-associated Enterobacter bacteremia and Klebsiella urinary tract infection, requiring administration of antibiotics and vasopressors (norepinephrine). She was intermittently febrile with temperature measured as high as 103.9°F. Due to prolonged intubation and requirement for tube feeding, tracheostomy, and percutaneous gastrostomy tube were placed.
There was mild transient elevation of serum alkaline phosphatase for 2 days, with a maximum value of 148 U/L, with other liver enzymes (AST and ALT) being elevated after the alkaline phosphatase peak. Serum creatine kinase was elevated up to 968 U/L around the same time. Serum calcium was decreased throughout the stay, with a minimum value of 7.3 mg/dL observed at the same time as the alkaline phosphatase peak. Serum phosphorus level and renal function remained within normal limits.
The patient was hospitalized for 47 days. She was discharged to a long term acute care facility. Tracheostomy and gastrostomy tubes were removed 1 month after discharge from the hospital, although she continued to require supplemental oxygen at 2 L/min via nasal cannula even 4 months after discharge. She underwent physical therapy and reported bilateral shoulder pain and stiffness following discharge. A chest radiograph obtained 4 months after discharge incidentally demonstrated HO around both shoulders, which was subsequently confirmed with bilateral shoulder radiographs and CTs (Fig 1, Fig 2, Fig 3, Fig 4, Fig 5). She was referred to an orthopedic surgeon with plan for surgical excision of the HO. The patient did not report symptoms of pain or stiffness in any other joints.
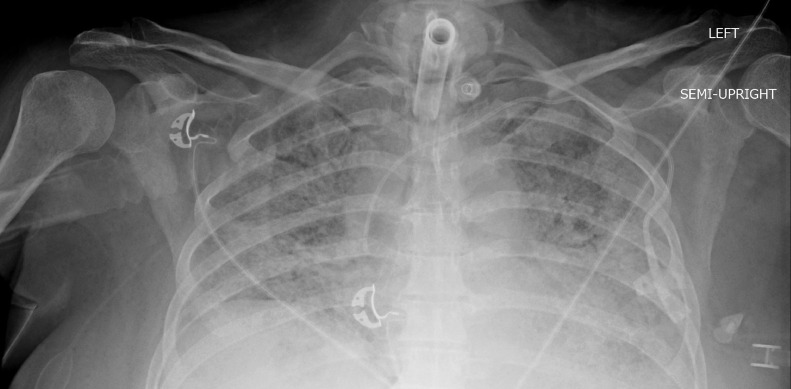
Fig 1.
Patient 1: Chest radiograph obtained 42 days after admission date demonstrates no evidence of HO around the shoulders. Tracheostomy tube, left subclavian approach central venous catheter, and bilateral pulmonary opacities compatible with infection/ARDS are noted.
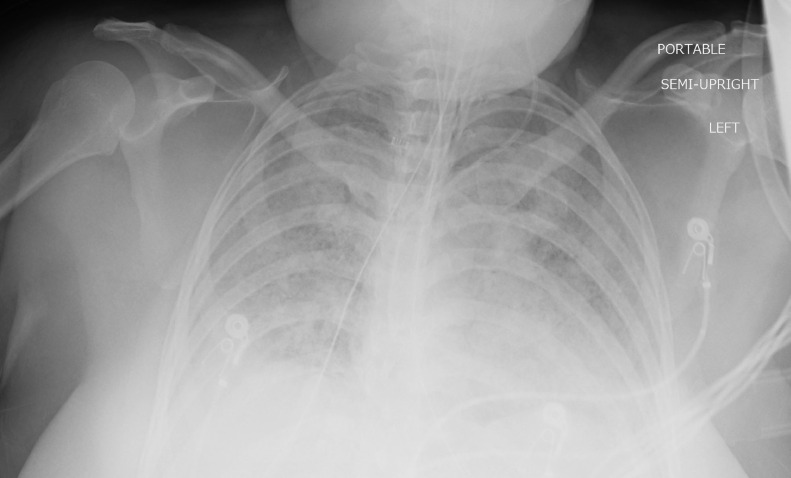
Fig 2.
Patient 1: Chest radiograph obtained 5.5 months post admission date. Interval development of HO around the bilateral glenohumeral joints. Interval removal of tracheostomy tube and central line, and clearing of previously seen bilateral pulmonary opacities.
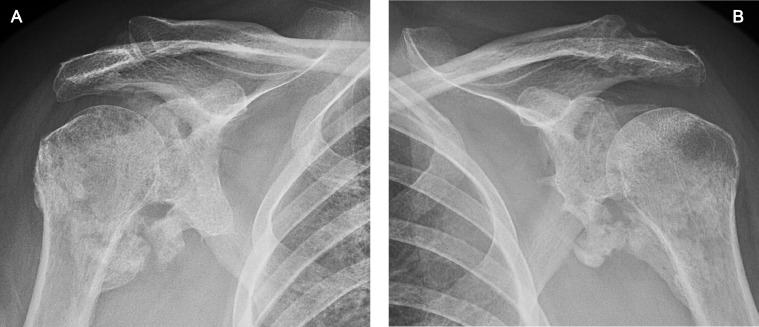
Fig 3.
Patient 1: AP radiographs of the (A) right and (B) left shoulders demonstrating mature HO around both glenohumeral joints, particularly inferiorly, with bridging or near-bridging. These radiographs were obtained 6 months post admission date.
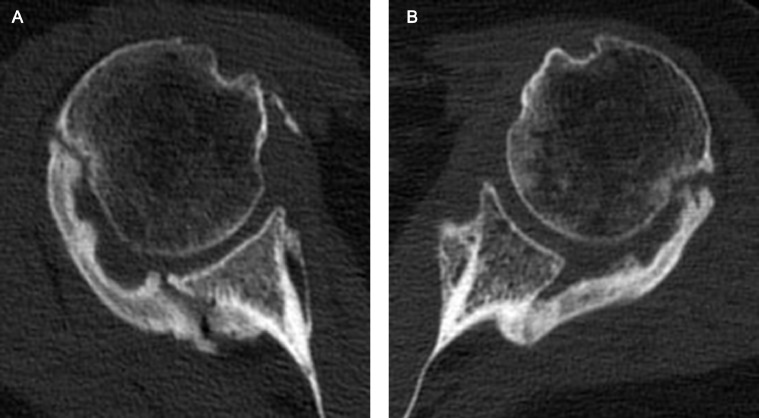
Fig 4.
Patient 1: Axial CT images of the (A) right and (B) left shoulders demonstrating near-bridging mature HO across the posterior aspects of the bilateral glenohumeral joints, with minimal HO anteriorly. The CTs were obtained 6.5 months post admission date.
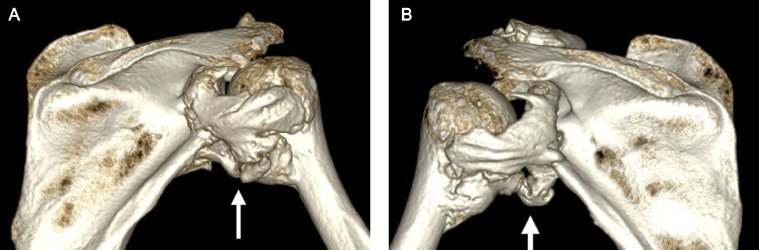
Fig 5.
Patient 1: Three-dimensional shaded surface displays of the CT of the (A) right and (B) left shoulders from a posterior perspective demonstrating near-bridging HO across the posterior and inferior aspects of the bilateral glenohumeral joints (arrows).
Patient 2
A 43-year-old female with past medical history of hypertension presented with shortness of breath, cough, and chest pain. She was diagnosed with COVID-19 by rapid antigen test from nasal swab. On admission, she required oxygen at 15 L/min with progression of symptoms requiring intubation and high FiO2 ventilation. Clinical and radiographic findings were consistent with infection/ARDS. Treatment included a single dose of tocilizumab, 4-day course of prednisone, 5-day course of ceftriaxone/doxycycline, and diuresis. Additional antibiotics were administered for presumed ventilator associated pneumonia. Supportive care included prone positioning. She suffered from septic shock requiring vasopressor support (norepinephrine). Fevers were recorded as high as 104.5°F.
Serum alkaline phosphatase remained within normal limits. Serum creatine kinase was markedly elevated up to 2199 U/L. Serum calcium was decreased throughout her hospital course with a minimum value of 7.7 mg/dL. Fluctuations in serum phosphorus level were seen, including values above and below the reference range. Renal function was decreased, with GFR down to 52.
The patient underwent tracheostomy 1 month after admission, and was subsequently discharged to a long term acute care facility 33 days after admission. About 5 months after discharge, the patient presented to the orthopedic clinic with right shoulder pain. Radiographs of the right shoulder revealed development of HO around the right glenohumeral joint without bony bridging, new since the time of hospitalization (Figs. 6 and 7). She was treated with a corticosteroid injection of the right glenohumeral joint and is currently undergoing physical therapy. The patient did not report significant pain or stiffness in any other joints.
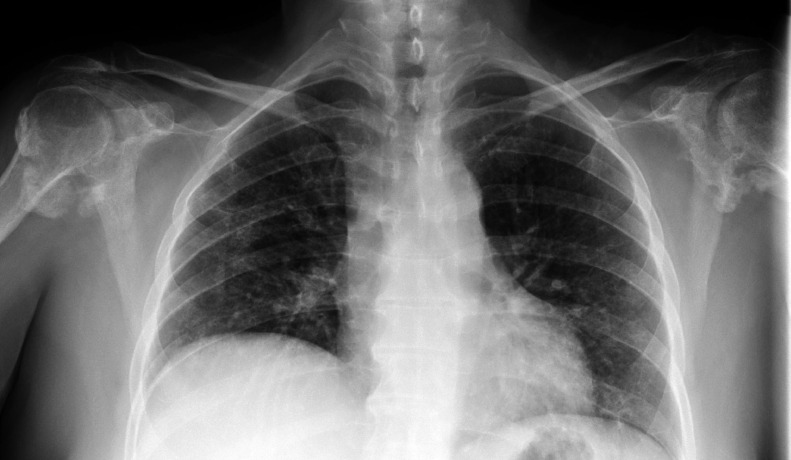
Fig 6.
Patient 2: Chest radiograph obtained 29 days after admission date demonstrates no evidence of HO around the shoulders, particularly the right shoulder. Endotracheal tube, enteric tube, left internal jugular approach central venous catheter, and bilateral pulmonary opacities compatible with infection/ARDS are noted.
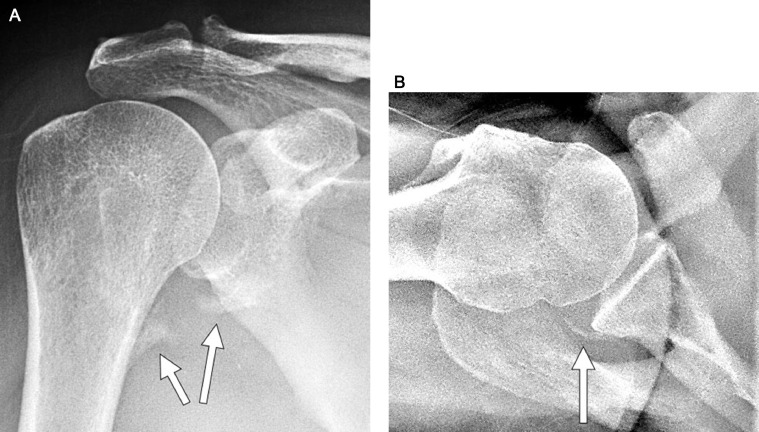
Fig 7.
Patient 2: (A) AP radiograph of the right shoulder demonstrating non-bridging HO around the inferior aspect of the glenohumeral joint (arrows). (B) Axillary view of the right shoulder demonstrating mild non-bridging HO around the posterior aspect of the glenohumeral joint (arrow). These radiographs were obtained 6 months post admission date.
Discussion
The pathophysiology of HO is multifactorial. HO is often incited by tissue injury, followed by an influx of inflammatory cells and subsequent downstream signaling events that inappropriately activate an osteogenic or osteochondrogenic pathway [1]. The pathways can be triggered by a variety of stimuli [3]. Established risk factors for HO include trauma, spinal cord injury, brain injury, arthroplasty, tissue hypoxia, immobilization with limitation of joint movement, hypermetabolic status, fever lasting more than 5 days, bone demineralization from prolonged bed rest, male sex, and age over 60 years [4]. Regional neurogenic, circulatory, metabolic, and biochemical changes have been implicated, as have genetic factors. Within the subset of metabolic disorders, alterations in calcium homeostasis may play a role in the development of HO. Similarly, the depletion or repletion of calcium during critical illness or bed rest may precipitate HO [5].
The common symptoms in HO are pain, swelling, warmth, and stiffness, which typically manifest 8-10 weeks after the initial insult. The most frequent physical exam finding is decreased range of motion in the affected joints. An acute rise in serum alkaline phosphatase and a transient depression in serum calcium may occur within the first 2 weeks [6]. Elevated alkaline phosphatase presumably reflects increased osteoblastic activity in the region of HO and has been associated with clinically significant HO [4]. However, alkaline phosphatase elevation can be seen with other entities resulting in increased bone turnover, such as skeletal trauma and bone metastases. Elevated creatine kinase may serve as marker for aggressive HO with extensive muscle involvement [7].
The development of HO after critical illness, prolonged paralysis, or immobilization must be recognized at an early stage to prevent functional loss. Alkaline phosphatase may serve as a useful screening tool, in addition to physical examination of proximal joints to assess range of motion [4]. Radiographs and CT are the primary imaging modalities for diagnosis of HO. Bone scan, ultrasonography, and MRI are sensitive for detection of early HO, although nonspecific. Bone scans may also be used to demonstrate quiescence of the HO prior to surgical excision [8].
Prophylaxis or treatment of HO can be done with radiotherapy, NSAIDs (particularly indomethacin) and bisphosphonates. After a long period of immobility, avoidance of aggressive mobilization has been suggested, as such local microtrauma may shear the soft tissues and provoke the pathogenesis of HO [9]. While most advise against passive exercises, which may exacerbate inflammation and potentiate HO, others recommend a physical therapy protocol to improve range of motion and limit contractures [1]. Therefore, clinical management in maturing HO differs.
In October 2020, Meyer et al described 4 cases of periarticular HO, 3 around the hip and 1 around the shoulder, in patients with recent history of COVID-19 with mechanical ventilation and prone positioning [2]. The 2 cases from our institution confirm that HO may be seen following COVID-19 critical illness. In our 2 cases, we suspect that chronic immobilization and hypoxia were involved in triggering HO. COVID-19 related restrictions may have limited mobilization of these patients by staff during hospitalization, resulting in greater immobility than occurs with other critical illnesses. It is possible that COVID-19 infection itself or novel treatment contributed to the pattern of HO, as global inflammation and the neuro-invasive potential of COVID-19 may incite HO [2]. The cytokine storm associated with COVID-19 includes upregulation of factors that have been previously associated with the formation of HO. Specifically, the coronavirus spike protein results in activation of the IL-6/TNF-α axis, causing elevated concentrations of these cytokines in critical COVID-19 patients; similar cytokines have been observed in the pathogenesis of HO [1,10]. Immunosuppressive drugs targeting these pathways in COVID-19 may therefore also theoretically inhibit HO, although further investigation is necessary.
Conclusion
Patients who have recovered from COVID-19 should be followed for symptoms of joint stiffness or pain. Such symptoms warrant imaging to evaluate for HO, with radiographs being the mainstay of diagnosis. Radiologists are advised to pay particular attention to the partially imaged shoulders on follow-up chest radiographs that may be obtained. It is unclear if HO can be prevented by prophylactic use of NSAIDS or bisphosphonates during hospitalization for COVID-19. Avoidance of aggressive physical therapy after prolonged immobilization has been suggested [1]. Steroid injections may help reduce pain. Further research is required to elucidate any specific relation between COVID-19 and the development of HO.
Footnotes
Formal consents are not required for the use of entirely anonymized images from which the individual cannot be identified - for example, xrays, ultrasound images, pathology slides or laparoscopic images, provided that these do not contain any identifying marks and are not accompanied by text that might identify the individual concerned.
References
- 1.Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4):e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer C, Haustrate MA, Nisolle JF, Deltombe T. Heterotopic ossification in COVID-19: a series of 4 cases. Ann Phys Rehabil Med. 2020;63(6):565–567. doi: 10.1016/j.rehab.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson SJ, Brett SJ. Heterotopic ossification - a long-term consequence of prolonged immobility. Crit Care. 2006;10(6):174. doi: 10.1186/cc5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, Collaco CR, Bruera E. Heterotopic ossification in critical illness and cancer: a report of 2 cases. Arch Phys Med Rehabil. 2002;83(6):855–859. doi: 10.1053/apmr.2002.32440. [DOI] [PubMed] [Google Scholar]
- 5.Clements NC, Jr, Camilli AE. Heterotopic ossification complicating critical illness. Chest. 1993;104(5):1526–1528. doi: 10.1378/chest.104.5.1526. [DOI] [PubMed] [Google Scholar]
- 6.Lane JE, Dean RJ, Foulkes GD, Chandler PW. Idiopathic heterotopic ossification in the intensive care setting. Postgrad Med J. 2002;78(922):494–495. doi: 10.1136/pmj.78.922.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman AL, Williams J, Patrick L, Banovac K. The value of serum creatine kinase in early diagnosis of heterotopic ossification. J Spinal Cord Med. 2003;26(3):227–230. doi: 10.1080/10790268.2003.11753688. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JW, De Sonnaville PB, Hulsmans HM, van Rinsum AC, Bijlsma JW. Polyarticular heterotopic ossification complicating critical illness. Rheumatology (Oxford) 1999;38(11):1145–1149. doi: 10.1093/rheumatology/38.11.1145. [DOI] [PubMed] [Google Scholar]
- 9.Christakou A, Alimatiri M, Kouvarakos A, Papadopoulos E, Patsaki I, Kotanidou A. Heterotopic ossification in critical ill patients: a review. Int J Physiother Res. 2013;1(4):188–195. https://www.ijmhr.org/ijpr_articles_vol1_4/333.pdf [Google Scholar]
- 10.Hussman JP. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front Pharmacol. 2020;11:1169. doi: 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]