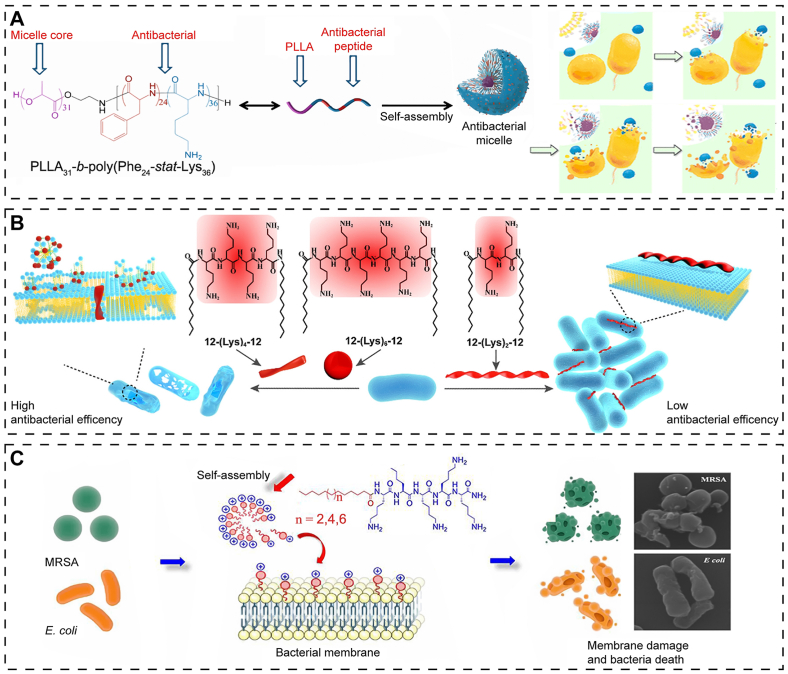
Fig. 2.
Structures and mode of antibacterial action of peptide amphiphiles. (A) Self-assembly of poly(l-lactide)31-b-poly(phenylalanine24-stat-lysine36)-based co-polypeptides into micelles with positive charges and amphipathic features, with PLLA segments aggregating to form the micellar core and with the polypeptide blocks serving as the corona of micelles. The cationic Lys units adhere to the bacterial membrane, and the hydrophobic Phe units enter and disrupt the bacterial membrane. Reproduced with permission from Ref. [53], copyright 2016, American Chemical Society. (B) C12-(lysine)n-C12 with diverse lysine spacer lengths form distinct supramolecular nanostructures: fibers (n = 2), rods (n = 4), and spherical aggregates (n = 6) that exhibit different antibacterial activities and action mechanisms. Reproduced with permission from Ref. [57], copyright 2018, American Chemical Society. (C) Micelle-forming cationic peptide amphiphiles, Cn+11-lysine5 (n = 2, 4, 6), demonstrate excellent antibacterial activity against methicillin-resistant Staphylococcus aureus and Escherichia coli. Reproduced with permission from Ref. [58], copyright 2018, American Chemical Society.