Figure 2.
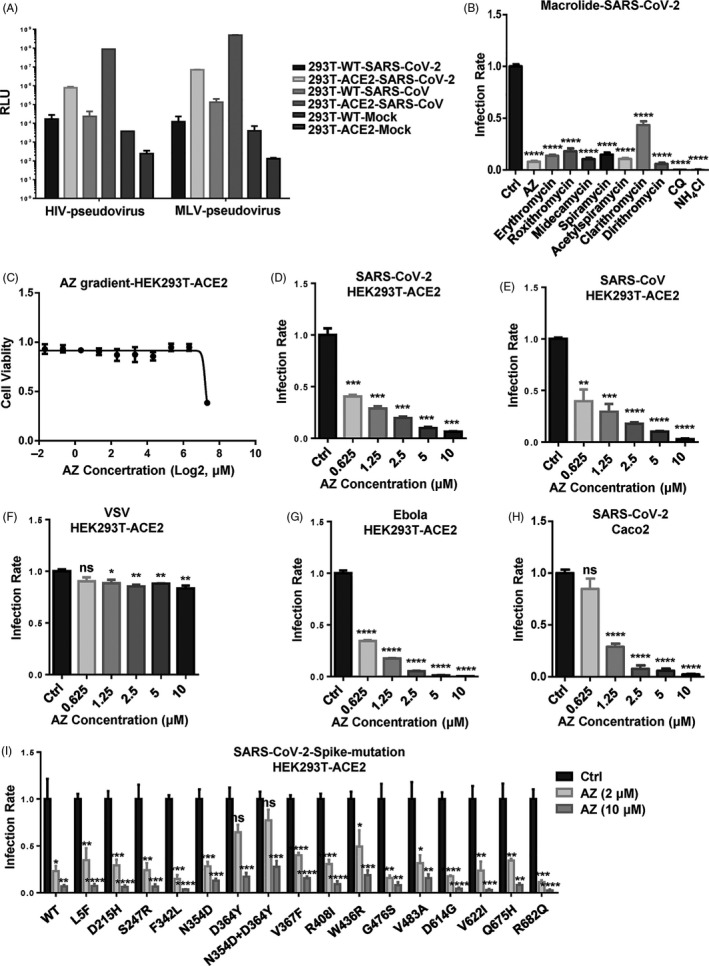
AZ has antiviral activity against SARS‐CoV‐2 pseudovirus in vitro. A, The infectivities of SARS‐CoV and SARS‐CoV‐2 pseudovirus in WT and human ACE2 stably expressed HEK293T cells. B, The relative inhibitory rate of AZ (10 μmol/L), erythromycin (10 μmol/L), roxithromycin (10 μmol/L), midecamycin (10 μmol/L), spiramycin (10 μmol/L), acetylspiramycin (10 μmol/L), clarithromycin (10 μmol/L), dirithromycin (10 μmol/L), chloroquine (CQ, 10 μmol/L) and NH4Cl (10 mmol/L) on SARS‐CoV‐2 pseudovirus infection in HEK293T‐ACE2 cells. C, The relative cell viability of HEK293T‐ACE2 cells treated with AZ (0.15625‐160 μmol/L) for 72 h. D‐G, The relative inhibitory rate on SARS‐CoV‐2, SARS‐CoV, VSV and Ebola pseudovirus infection in HEK293T‐ACE2 cells treated with AZ (0.625‐10 μmol/L). H, The relative inhibitory rate on SARS‐CoV‐2 pseudovirus infection in Caco2 cells treated with AZ (0.625‐10 μmol/L). I, The relative inhibitory rate on WT and mutant SARS‐CoV‐2 pseudovirus infection in HEK293T‐ACE2 cells treated with AZ (2 and 10 μmol/L). Solvent was treated as Ctrl. Experiments were repeated twice. ns, no significant, *P < .05, **P < .01, ***P < .001, ****P < .0001
