Abstract
Kidney transplant (KT) recipients are at an increased risk for severe COVID‐19 because of their immunosuppressed state. A 42‐year‐old KT patient was diagnosed with COVID‐19 three months after KT. Despite lymphopenia and several risk factors, he had a mild disease course. Nasopharyngeal real‐time reverse transcriptase polymerase chain reaction for SARS‐CoV‐2 became negative 48 days after detection. SARS‑CoV‑2 IgG antibodies became negative after day 40. TTV DNA load increased with the onset COVID‐19 and reduced after its resolution. This is the first report where TTV DNA load was measured during the course of COVID‐19.
Keywords: COVID‐19, kidney transplant, SARS‐CoV‐2, torquetenovirus
1. INTRODUCTION
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged as the etiologic agent of Coronavirus disease 2019 (COVID‐19). 1 COVID‐19 has different degrees of severity. Older adults and patients with chronic diseases are at a higher risk of developing severe disease. 2
Testing for SARS‐CoV‐2 RNA remains the gold standard for COVID‐19 diagnosis, 3 however, SARS‐CoV‐2 IgG/IgM antibodies may be relevant for viral clearance. 4
Recently, several nationwide and multicentric studies concerning COVID‐19 and kidney transplantation (KT) have been published in the literature. These studies have primarily focused on clinical and laboratory risk factors for severe disease and mortality. 5 , 6 , 7 Kidney transplant recipients are at an increased risk for severe COVID‐19 because of their immunosuppression. Conversely, as severe disease results from a hyper‐inflammatory state, immunosuppression may be beneficial. 8 , 9
No ideal marker reliably defines the immune function of KT patients. Torquetenovirus (TTV) has recently gained attention as a potential surrogate marker of the net state of immunosuppression. 10 The inverse correlation between immune competence and TTV replication might be a promising strategy.
We report a mild course of SARS‐CoV‐2 infection with prolonged viral shedding and failed antibody response in a recent KT recipient. TTV DNA load increased with the onset COVID‐19 and reduced after its resolution.
1.1. Case report
A 42‐year‐old man with end‐stage renal disease because of diabetic nephropathy received a KT from a non‐heart‐beating donor in January 26, 2020. Obesity and hypertension were additional comorbidities.
Immunosuppression included thymoglobulin, tacrolimus, mycophenolate mofetil (MMF), and prednisolone. Hemodialysis was required for 2 weeks after KT because of delayed graft function. Kidney function gradually improved and his eGFR (CKD‐EPI) at discharge was 36 mL/min/1.73 m2.
On April 25, 2020 (day 0) he was admitted for elective removal of ureteral stent. He complained of low‐grade fever and mild thoracic pain 3 days prior to admission. He denied dyspnea, cough, or gastrointestinal symptoms. Physical evaluation was unremarkable: body temperature was 36.5ºC and oxygen saturation was 98% in ambient air, blood pressure was 110/69 mmHg and respiratory rate was 25 breaths per minute. Real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) nasopharyngeal swab for SARS‐CoV‐2, routinely performed 24 hours before surgical procedures, unveiled a positive result. Laboratory results revealed lymphopenia, slightly elevated C‐reactive protein and D‐dimer, stable kidney function (Figure 1) and normal levels of transaminases, lactic dehydrogenase, and ferritin. Tacrolimus through blood level of tacrolimus was 10.6 ng/mL. Arterial blood gas exam and chest X‐ray were normal.
Figure 1.
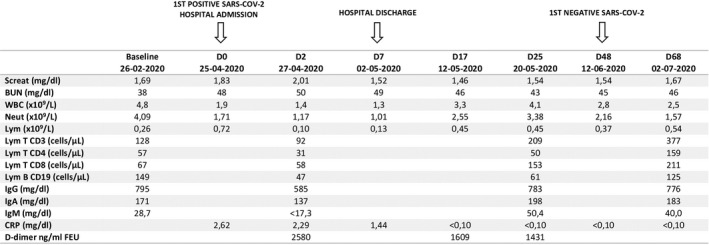
SARS‐CoV‐2: Severe acute respiratory syndrome Coronavirus 2; Screat: seric creatinine; BUN: blood urea nitrogen; WBC: white blood cells; Neut: neutrophils; Lym: lymphocytes; IgG: immunoglobulin G; IgA: immunoglobulin A; IgM: immunoglobulin M; CRP: C‐reactive protein; FEU: fibrinogen equivalent units; 1st: first
He was admitted to a COVID‐19 specific ward. On admission, tacrolimus dose was reduced, prednisolone was increased to 20 mg/day and MMF was suspended. On day 2, TTV viral load was 7.14log10, serum Immunoglobulin G (IgG) and Immunoglobulin M (IgM) were decreased and CD4+, CD8+, CD3+, and CD 19 + count in peripheral blood were reduced ( Figure 1 ). Cytomegalovirus (CMV), BK virus (BKV), and JC virus (JCV) viremia were absent.
During admission, he remained asymptomatic with stable renal function but with persistent leucopenia and lymphopenia. No antimicrobial or antiviral therapies were prescribed. He was discharged at day 7.
After discharge, lymphopenia, IgG, and IgM levels progressively improved, but lymphocyte subpopulations remained reduced on day 25 (Figure 1 ). MMF was restarted (250 mg two times a day) at day 17. BKV, JCV, and CMV viremia remained undetectable along the course of COVID‐19.
Total antibodies (Ab) (IgM/IgG) and specific IgG antibodies against SARS‐CoV‐2 were performed on day 17, 25, 40, and 48. Titers of SARS‑CoV‑2 total Ab were negative in all four determinations. SARS‐CoV‐2 IgG antibodies were positive on day 17 and 25 and became negative after day 40 (Figure 2 ).
Figure 2.
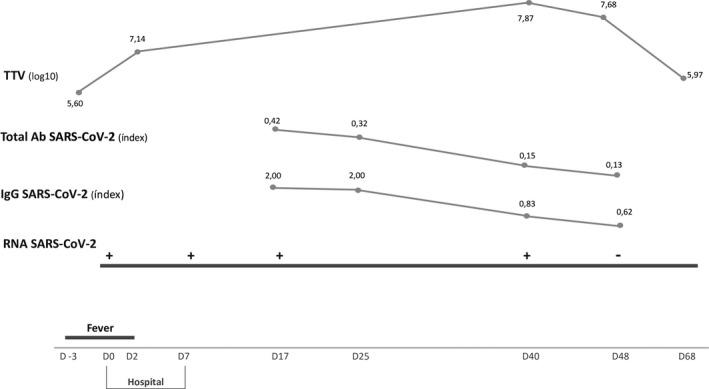
TTV: torquetenovirus; Ab: antibodies; SARS‐CoV‐2: Severe acute respiratory syndrome Coronavirus 2; IgG: immunoglobulin G; RNA: ribonucleic acid. Total Ab SARS‐CoV‐2 ‐ Electrochemiluminescence immunoassay for qualitative in vitro detection of total antibodies (IgM and IgG) against SARS‑CoV‑2 (Elecsys Anti‐SARS‑CoV‑2 total Ab, Cobas e602); negative < 1. IgG SARS‐CoV‐2 ‐ Chemiluminescence microparticle immunoassay for qualitative detection of IgG antibodies against SARS‐CoV‐2 (SARS‐CoV‐2 IgG, ARCHITECT i System); negative < 1.4
RT‐PCR SARS‐CoV‐2 became negative on day 48 and day 50, when he was considered cured.
Kinetics of TTV DNA load was stable during COVID‐19 (7.14 log10‐7.87 log10), however, 2log10 higher than TTV viral load at month 1 and at month 6 after KT (5.6 log10 and 5.9 log10, respectively) (Figure 2 ).
2. DISCUSSION
We present a patient infected with SARS‐CoV‐2 3 months after a KT. Despite several risk factors for severe COVID‐19 (immunosuppression, diabetes, hypertension, and obesity) and biomarkers associated with poor outcomes (neutropenia, lymphopenia, elevated levels of C‐reactive protein and D‐dimer), he had a mild disease course. Lymphocyte subpopulations were also reduced, consistent with an immunosuppressed status.
Lymphopenia is a common feature in patients with COVID‐19 and may be a critical factor associated with disease severity and mortality. 11 In the setting of transplantation, lymphopenia may be multifactorial. As many KT patients have pre‐existing lymphocyte depletion, further drop in lymphocyte may be more meaningful as a prognostic marker. 12 In our patient, lymphopenia was present before COVID‐19, because of thymoglobulin induction and maintenance immunosuppression. Nevertheless, lymphocytes significantly decreased between D2 and D7, with subsequent partial recovery.
Managing immunosuppression in KT patients infected with SARS‐CoV‐2 should take into account severity of disease, age, comorbidities, and time post‐transplant. Although reduction or temporary discontinuation of immunosuppression has been recommended in KT patients with COVID‐19, 13 withdrawal of immunosuppression may favor SARS‐CoV‐2 activated cytokine storm and increases the risk of rejection.
Shingare A et al 14 described 2 KT recipients who developed mild COVID‐19 in the first month after transplantation. Both received anti‐thymocyte globulin and developed lymphopenia. Antimetabolite was interrupted in one patient and reduced in the other; no allograft dysfunction was observed in either case.
In our patient, antimetabolite was discontinued because of neutropenia and lymphopenia rather than to the clinical course of disease. However, it is not clear if early MMF discontinuation influenced clinical outcomes.
At admission, the dose of prednisolone was increased from 5 mg to 20 mg/day, with progressive tapering. Steroid increment may be justified to reduce the risk of acute rejection, suppress the inflammatory cascade and reduce mortality. 15 However, the additional risk of opportunistic infections needs to be weighed. In this case, despite adjustment in immunosuppression, kidney function remained stable without evidence of rejection.
In non‐immunosuppressed patients, age is the main contributor to disease severity and outcome, 16 so this could be one of the reasons for the mild course of disease in this case.
In general population with COVID‐19, the median duration of virus shedding is 20 days. 17 However, in solid organ transplant recipients viral shedding could last longer, up to 68 days. 14 , 17 Our patient presented viral shedding for 48 days after diagnosis. Immunosuppression may impair viral clearance, 18 leading to a prolonged viral shedding.
Data on antibody response in transplant patients are scarce. In a recent report, 19 a KT patient who developed COVID‐19 pneumonia less than one month after KT, did not develop IgM/IgG antibodies for more than 2 months. In our report, SARS‑CoV‑2 total Ab were sustainably negative, probably because of an inability of suppressed naïve T cells to recognize viral antigens, contributing to absent response of specific humoral immunity. 20 However, IgG antibodies were marginally positive but became negative after day 40. Possible explanations include false positivity or viral‐induced clonal deletion. Reduced total IgM levels, borderline low IgG levels and reduced CD19 + lymphocytes during COVID‐19, reveal an impaired humoral immunity, which could contribute to the inability to produce a specific antibody response against SARS‐CoV‐2.
Until now, no reliable marker has been identified to quantify the net state of immunosuppression in transplant patients. Kinetics of TTV DNA load has gained attention and have made this virus a possible marker of immune function, able to predict the risk of graft rejection and infection after KT. 10 It is expected that TTV viral load increases from day 7 do day 30 after KT, peaks around month 3 to 12 post‐KT and reaches steady state thereafter. 21 In a recent study, 21 TTV DNA loads above 3.15 log10 and 4.56 log10 copies/mL at month 1 predicted the occurrence of post‐transplant infection (adjusted hazard ratio [aHR]: 2.88; 95% confidence interval [CI]: 1.13‐7.36; P‐value = .027). In another study including 386 KT patients, 22 TTV viral load was higher at the end of month 3 post‐transplant and reached steady state thereafter. Authors defined a TTV load between 1 × 106 and 1 × 108 copies/mL as the optimal range to minimize the risk for rejection and infection.
Our patient was included in a prospective cohort study where TTV levels are measured at KT time, 1 week, 1 month and 3 months after KT and then quarterly until the first year post‐KT. Coincidently, COVID‐19 was diagnosed at the third month after KT. TTV DNA load was stable during COVID‐19 (7.14log10‐7.87 log10), but higher than the TTV DNA load at month 1 and at month 6 after KT (5.6 log10 and 5.9 log10, respectively). This increase in 2log10 during the course of COVID‐19 is in line with the expected kinetics of TTV DNA load along the third month after KT. Regarding this information, it seems that a mild COVID‐19 course does not change the net state of immunosuppression. Nevertheless, a role of COVID‐19 on the increase in TTV DNA load cannot be excluded.
Considering the optimal range of TTV viral load, between 6 log10 and 8 log10, the risk of organ rejection or severe infection during this period was accordingly minimized in this patient, in line with the good outcome of SARS‐CoV‐2 infection.
This is the first report where the kinetics of TTV DNA load was measured during COVID‐19. Further studies are needed to describe the kinetics of TTV viral load as a marker of immune function in KT patients infected with SARS‐CoV‐2, as well as in the general population.
CONFLICT OF INTEREST
None. The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHORS’ CONTRIBUTIONS
S. Querido: concept/design, research study, data collection, data analysis, bibliographic review, drafting article; R. Calça and D. Francisco: chart review, data analysis; A. Weigert: drafting article and critical revision of article; T. Adragão and D. Machado: critical revision of article; MA Pessanha, P. Gomes, L. Rodrigues, J. M. Figueira and C. Cardoso: data collection, laboratorial analysis and critical revision of article.
Querido S, Calça R, Weigert A, et al. Kinetics of torquetenovirus DNA load in a recent kidney transplant recipient with mild SARS‐CoV‐2 infection and a failed antibody response. Transpl Infect Dis.2021;23:e13524. 10.1111/tid.13524
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific COVID‐19‐RdRp/Hel real‐time reverse transcription‐PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310 ‐ e00320. Published 2020 Apr 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du Z, Zhu F, Guo F, Yang B, Wang T. Detection of antibodies against SARS‐CoV‐2 in patients with COVID‐19. J Med Virol. 2020;92(10):1735–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig‐Schapiro R, Salinas T, Lubetzky M, et al. COVID‐19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2020; 10.1111/ajt.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma P, Chen V, Fung CM, et al. COVID‐19 outcomes among solid organ transplant recipients: a case‐control study. Transplantation. 2021;105(1):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elias M, Pievani D, Randoux C, et al. COVID‐19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31(10):2413‐2423. 10.1681/ASN.2020050639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832‐834. [DOI] [PubMed] [Google Scholar]
- 10. Rezahosseini O, Drabe CH, Sørensen SS, et al. Torque‐teno virus viral load as a potential endogenous marker of immune function in solid organ transplantation. Transplant Rev (Orlando). 2019;33(3):137‐144. [DOI] [PubMed] [Google Scholar]
- 11. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kronbichler A, Gauckler P, Windpessl M, et al. COVID‐19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16(7):365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shingare A, Bahadur MM, Raina S. COVID‐19 in recent kidney transplant recipients. Am J Transplant. 2020;20(11):3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahase E. Covid‐19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. Published 2020 Jun. [DOI] [PubMed] [Google Scholar]
- 16. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 xases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 17. Man Z, Jing Z, Huibo S, Bin L, Fanjun Z. Viral shedding prolongation in a kidney transplant patient with COVID‐19 pneumonia. Am J Transplant. 2020;20(9):2626–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fishman JA, Grossi PA. novel coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20(7):1765‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia Z, Liu X, Hu X, et al. Failed antibody response in a renal transplant recipient with SARS‐CoV‐2 infected. Transpl Infect Dis. 2020;22(5): e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775–1776. [DOI] [PubMed] [Google Scholar]
- 21. Fernández‐Ruiz M, Albert E, Giménez E, et al. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression‐related complications after kidney transplantation. Am J Transplant. 2019;19(4):1139. [DOI] [PubMed] [Google Scholar]
- 22. Doberer K, Schiemann M, Strassl R, et al. Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients‐A prospective observational trial. Am J Transplant. 2020;20(8):2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]


