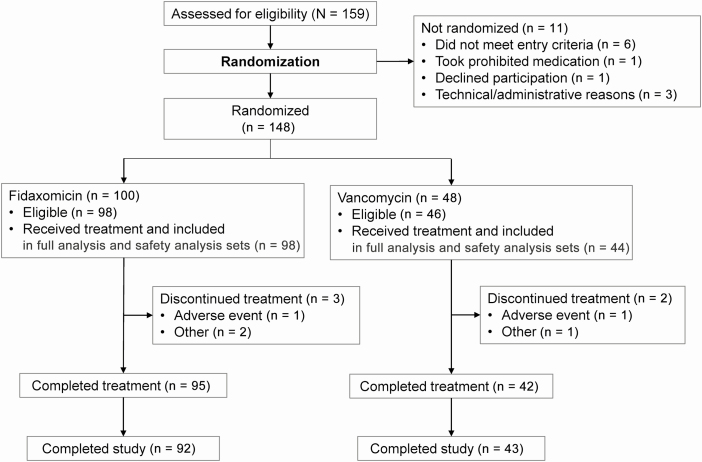
Figure 2.
Patient flow through the study. Of the 2 patients randomized to fidaxomicin who did not receive treatment, 1 did not meet the diagnostic criteria for Clostridioides (Clostridium) difficile infection (CDI), and 1 did not meet the diagnostic criteria for CDI and had received an investigational therapy ≤28 days before screening. Of the 4 patients randomized to vancomycin who did not receive treatment, 2 entered the study although they did not satisfy entry criteria owing to a history of inflammatory bowel disease, 1 eligible patient withdrew consent, and 1 eligible patient did not receive the study drug. Five patients in the fidaxomicin arm completed treatment but did not complete the study. Two patients in the fidaxomicin arm and 1 in the vancomycin arm did not complete treatment but completed the study.