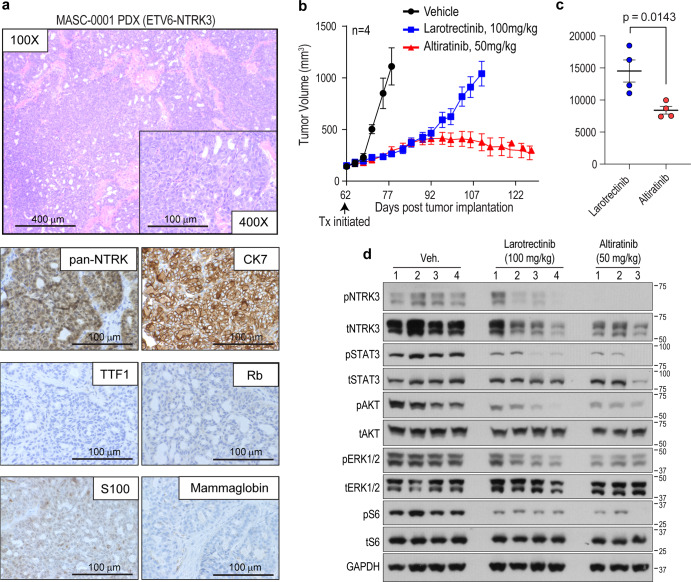
Fig. 3. Altiratinib inhibits growth of ETV6-NTRK3G623R patient-derived xenograft tumors.
a Morphological and immunohistochemical characterization of patient-derived xenograft (PDX) that was established from tumor samples obtained from a MASC patient (MASC-0001) harboring the ETV6-NTRK3 fusion and who developed the G623R mutation after entrectinib treatment. b Mice bearing PDX tumors were treated with larotrectinib (100 mg/kg, twice daily (bis in die, (BID), shown in blue) or altiratinib (50 mg/kg BID, shown in red) as compared to vehicle-treated tumor-bearing mice (vehicle, BID, shown in black). Treatment (Tx) was initiated 62 days post implantation (black arrow) when tumor volume was ~100–200 mm3. Tumor volume measurements are shown as a function of time. c Area under the curve analysis compares the efficacy of larotrectinib and altiratinib tumors. Average ± standard error of means (SEM) is shown with statistical significance indicated by p value (unpaired Student’s t-test). d Immunoblot analysis of lysates generated from treated tumors (from (b)). Phosphorylation of ETV6-NTRK3 (pNTRK3), STAT3 (pSTAT3), AKT (pAKT), ERK1/2 (pERK1/2), and ribosomal protein S6 (pS6), as well as corresponding total levels of these proteins and GAPDH (loading control). “Veh.” indicates vehicle (DMSO) treatment. Numbers above blots represent separate tumors from individual mice with n = 4 for the vehicle and larotrectinib groups, and n = 3 for the altiratinib group. There was insufficient material from the 4th altiratinib-treated tumor for biochemical analysis.