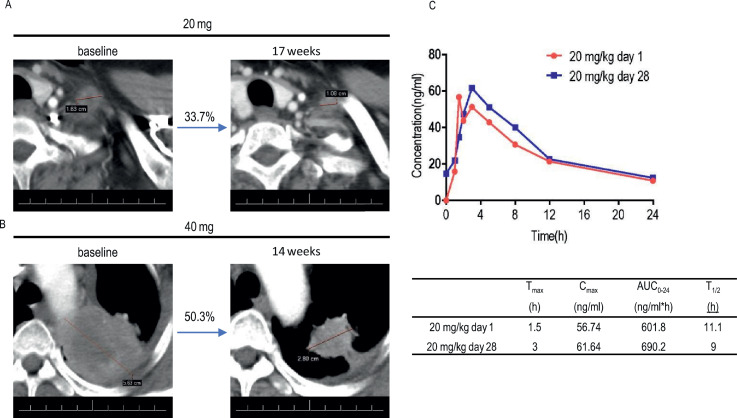
Fig. 7.
Tumor response assessments and preliminary pharmacokinetic profile of BEBT-109 in patients with NSCLC harboring EGFR T790M mutation in a first-in-human dose-escalation study. A, Computed tomographic scan images of metastatic region in left supraclavicular lymph nodes from patient with NSCLC harboring EGFR L858R T790M R831H mutation before and after BEBT-109 treatment in phase I clinical trial. The patient was previously treated with Icotinib and followed by 6 cycles of pemetrexed plus carboplatin. Patient was administered BEBT-109 at a dose of 20 mg once per day, and tumor shrinkage of 33.7% was observed. Lesions are outlined by a red line. B, Computed tomographic scan images of left upper lobe from patient with NSCLC harboring EGFR Del19 T790M mutation before and after BEBT-109 treatment in phase I clinical trial. The patient was previously treated with AZD3759. Patient was administered BEBT-109 40 mg/kg/day, and tumor shrinkage of 50.3% was observed. Lesions are denoted by a red line. C, Preliminary pharmacokinetic profiles from the patient in the 20 mg/day group at day 1 and day 28.