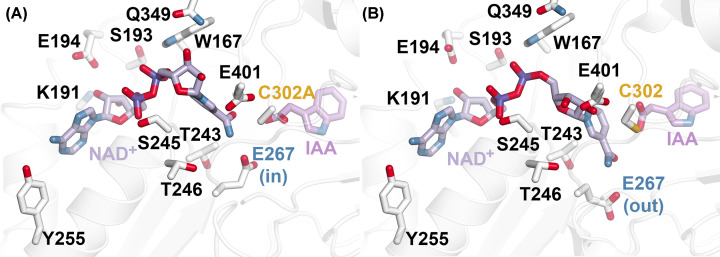
Figure 3. NAD+ isomerization in AldA.
(A) NAD+ binding in the AldA C302A X-ray crystal structure. The nicotinamide half of the ligand adopts a conformation that positions the nicotinamde ring away from the catalytic site (i.e., C302A in gold), which is ideal for the hydrolysis step of the reaction. The side-chain of Glu267 (blue; in) is positioned into the active site. (B) NAD+ binding in the AldA wild-type X-ray crystal structure. As previously reported [18], the nicotinamide half of the ligand adopts a conformation that positions the nicotinamde ring into the catalytic site (i.e., C302 in gold), which is ideal for the hydride transfer step of the reaction. The side-chain of Glu267 (blue; out) is positioned away from the active site.