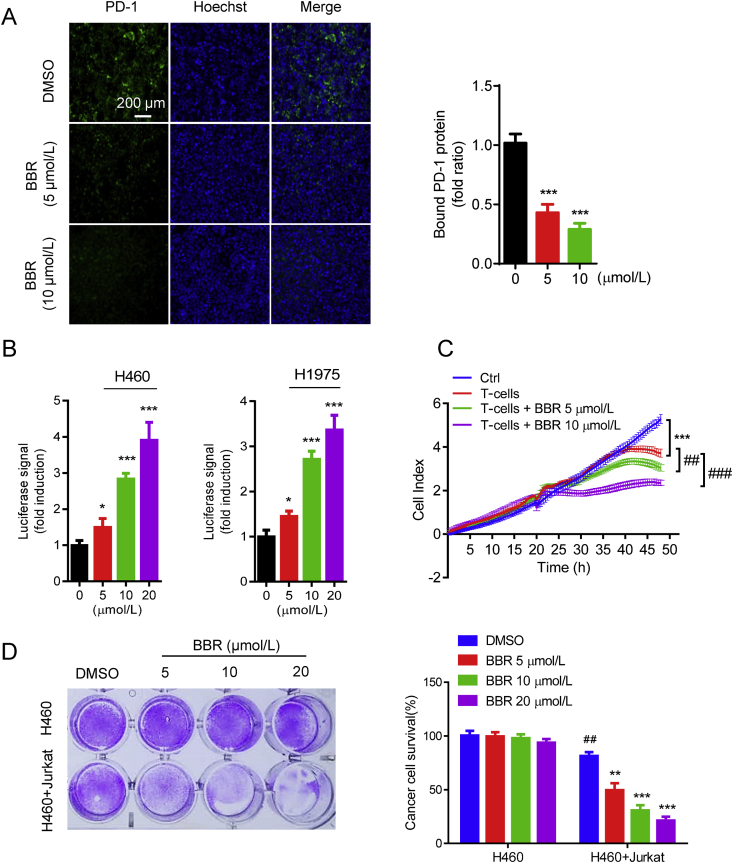
Figure 2.
BBR recovers the cytotoxicity of T-cells. (A) PD-L1/PD-1 binding assay in H157 cells treated with BBR (5 and 10 μmol/L, 24 h). The nuclei were stained with Hoechst. Scale bar, 200 μm. Bound PD-1 was calculated according to the intensity of green fluorescence (n = 3). ∗∗∗P < 0.001 compared with DMSO group. (B) PD-L1/PD-1 blockade assay performed with H460 and H1975 cells treated with 5, 10, and 20 μmol/L BBR for 16 h. Jurkat NFAT-luciferase reporter cells (10,000 cells/well) were added, and cells were co-cultured for 6 h. Data are presented as fold induction over untreated control (n = 3). ∗P < 0.05, ∗∗∗P < 0.001 compared with DMSO group. (C) Cell impedance assay analyzing human T-cell (activated by anti-CD3 plus anti-CD28 co-stimulation)-meditated tumor cell killing in H460 cells treated with BBR (5 or 10 μmol/L) for 24 h (n = 3). ∗∗∗P < 0.001 compared with control, ##P < 0.01, ###P < 0.001 compared with T-cell only group. (D) Jurkat cells were activated by 1 mg/mL phytohemagglutinin (PHA) plus 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and co-cultured with H460 cells in 12-well plates for 2 days in the presence of BBR and the surviving tumor cells were visualized by crystal violet staining. Relative fold ratios of surviving cell intensity are shown (n = 3). ##P < 0.01 compared with H460 DMSO group; ∗∗P < 0.01, ∗∗∗P < 0.001 compared with H460+Jurkat DMSO group. Data shown are mean value of three independent experiments±standard error of mean (SEM).