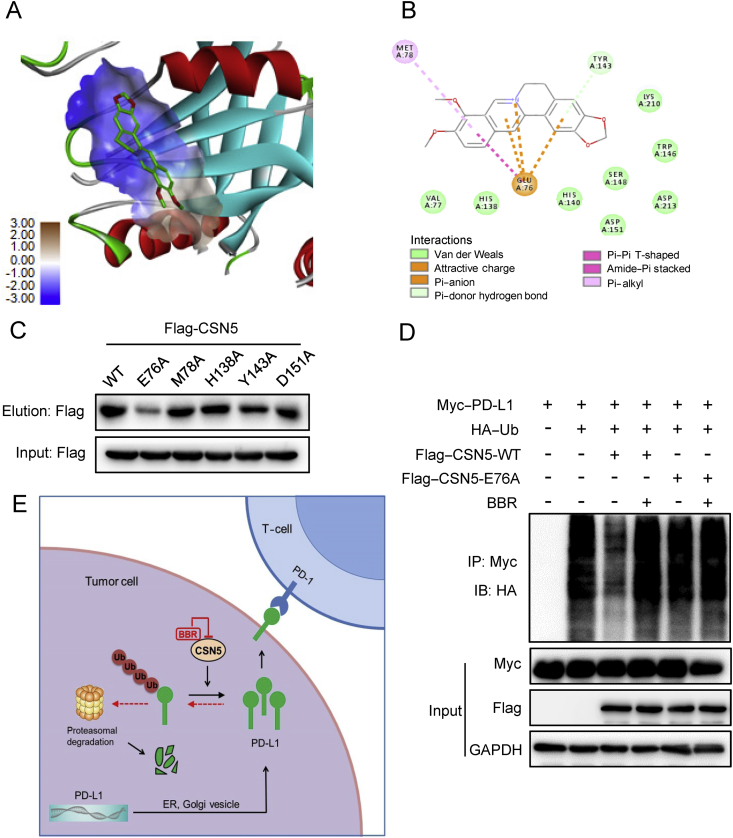
Figure 7.
Glu76 of CSN5 is critical for binding to BBR. (A) Molecular docking model carried out by Discovery Studio 4.5 revealed that BBR binds to the JAMA domain of CSN5. (B) BBR forms an ionic bond with the backbone of Glu76 and interacts favorably with several residues including Met78, His138, Tyr143, and Asp151. (C) 293T-cells were transfected with WT-CSN5 and CSN5-mutant plasmids for 48 h, cell lysates were incubated with BBR–biotin at 37 °C for 2 h, followed by pull-down with streptavidin–agarose; the precipitates were then immunoblotted by flag antibody. (D) 293T-cells were co-transfected with Myc–PD-L1, HA–Ub and Flag–CSN5 WT or Flag–CSN5 E76A, followed by BBR treatment for 8 h. The ubiquitination of PD-L1 were analyzed by IB. (E) Proposed model of BBR-mediated PD-L1 degradation. BBR specific binds to and subsequently inactivates CSN5, which led to degradation of PD-L1 and activation of tumor-infiltrating T-cells.