Extended Data Figure 6: KSR and RAF share complementary regulatory roles as MEK scaffolds and activators.
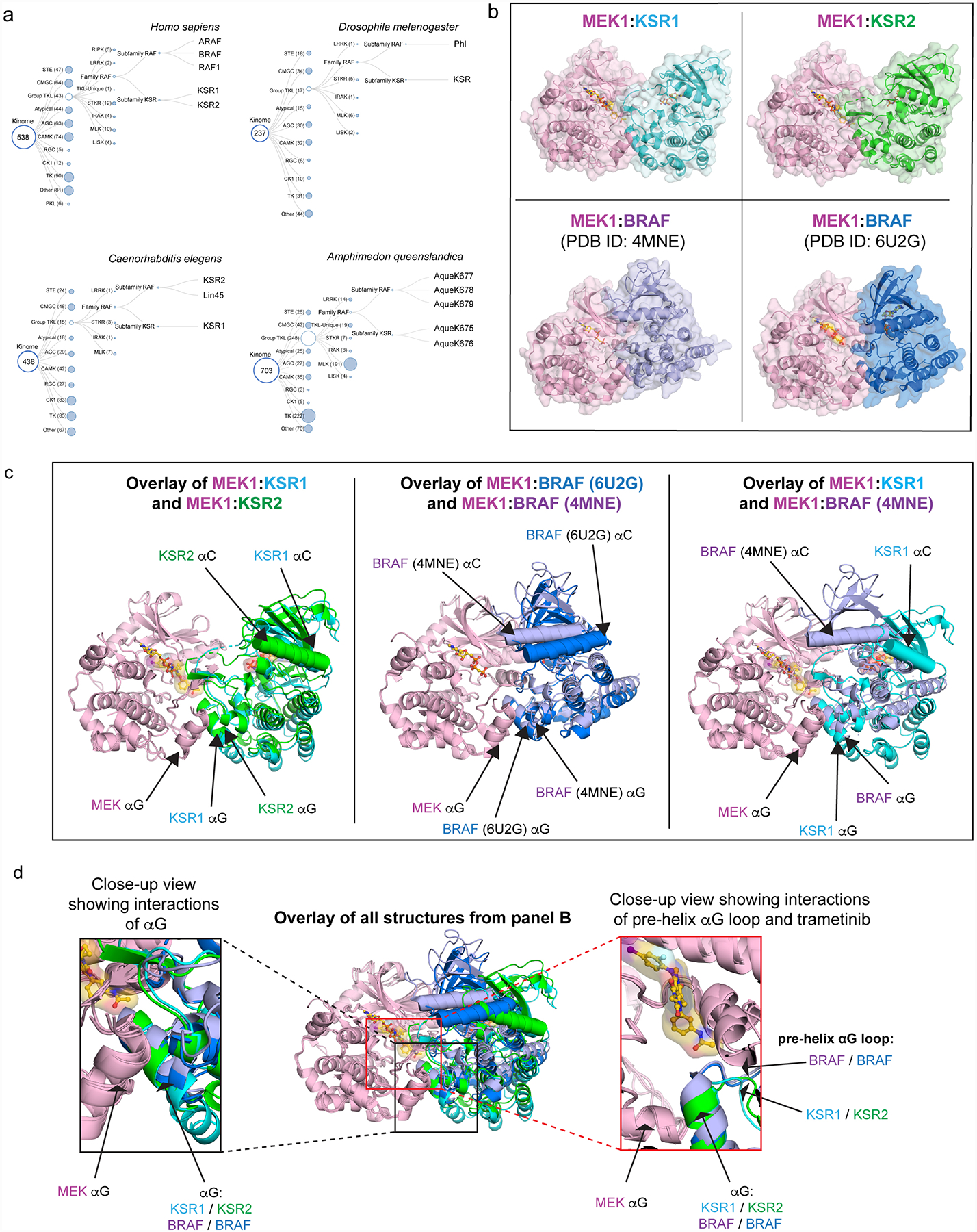
A. KSR and RAF family members appear to have co-evolved. Phylogenetic tree diagrams for the indicated species were generated from reported kinome sequence data that can be found at http://kinase.com/web/current/kinbase/. All species that we analysed include at least one RAF and one KSR homolog.
B. Structures of MEK1 in complex with KSR1 and KSR2 determined here, and previously determined structures of MEK1:BRAF-active conformation (PDB ID: 4MNE), and MEK1:BRAF-inactive conformation (PDB ID: 6U2G).
C. Structural overlay of MEK1-associated complexes highlights variations in the quaternary arrangements of KSR-bound MEK and RAF-bound MEK. Shown are overlays of MEK1:KSR1 with MEK1:KSR2 (left); MEK1:BRAF (PDB ID: 4MNE) with MEK1:BRAF (PDB ID: 6U2G) (center); and MEK1:KSR1 with MEK1:BRAF (PDB ID: 4MNE). In particular, the N-lobe, including helix αC, in KSR and RAF proteins are significantly displaced between distinct complexes. However, in contrast, the lower C lobe, including helix αG, appears relatively fixed in all sets of complexes.
D. Overlay of all structures, using MEK1 C-lobe as an anchor (center), demonstrates helix αG as a common docking site for reciprocal kinase domain interactions between MEK and BRAF or KSR (left inset). Further, the pre-helix αG loop regions within BRAF and KSR proteins occupy a relatively fixed location relative to MEK (right inset).
