Figure 3: The trametinib binding site distinguishes KSR from RAF.
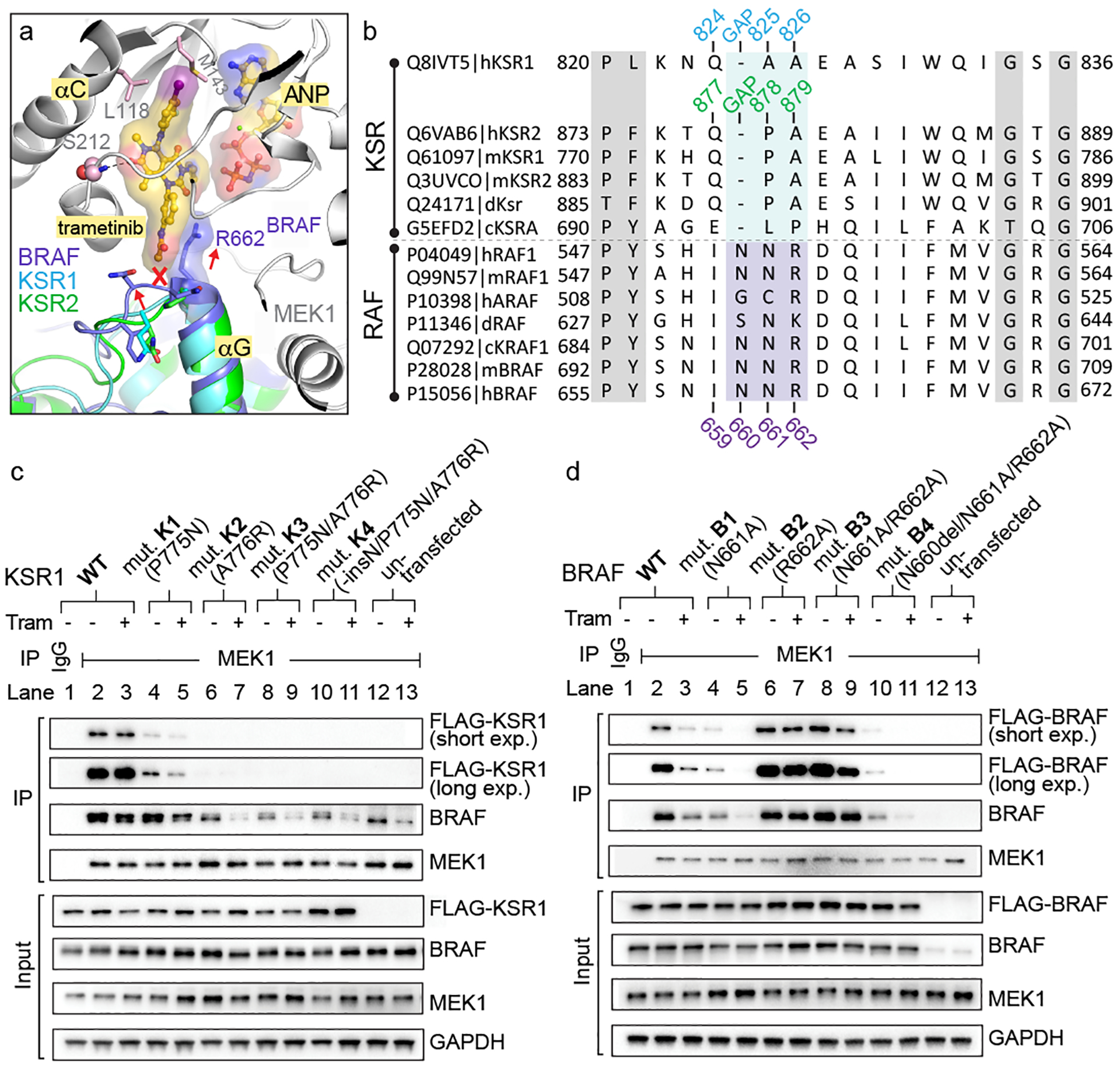
A. Structural overlay of the BRAF:MEK1, KSR1:MEK1, and KSR2:MEK1 complexes predicts a clash between trametinib and the pre-helix αG loop of BRAF.
B. Sequence alignment of RAF kinases and KSR pseudokinases at the trametinib-binding site. Numbering for human KSR1, KSR2, and BRAF highlighted.
C. And D.
IP/WB of endogenous MEK1 from lysates of HCT116 cells transfected with FLAG-tagged wild-type and mutant versions of full-length mouse KSR1 (panel C) or human BRAF (panel D). Cells were treated with DMSO or 200 nM trametinib for 1 hour prior to harvesting cells. IgG was used as a control for non-specific binding of proteins during IPs (lanes 1 vs 2). Transfected KSR1 and BRAF were detected using anti-FLAG antibody. All other western blot signals were detected using specific antibodies against endogenous proteins; note, the antibody against BRAF detects both endogenous and transfected FLAG-tagged BRAF. Blots are representative of three independent experiments with similar results, uncropped blots in Supplementary Figure 1.
