Figure 4: Trametiglue targets both KSR-MEK and RAF-MEK with unprecedented potency and selectivity via unique interfacial binding interactions.
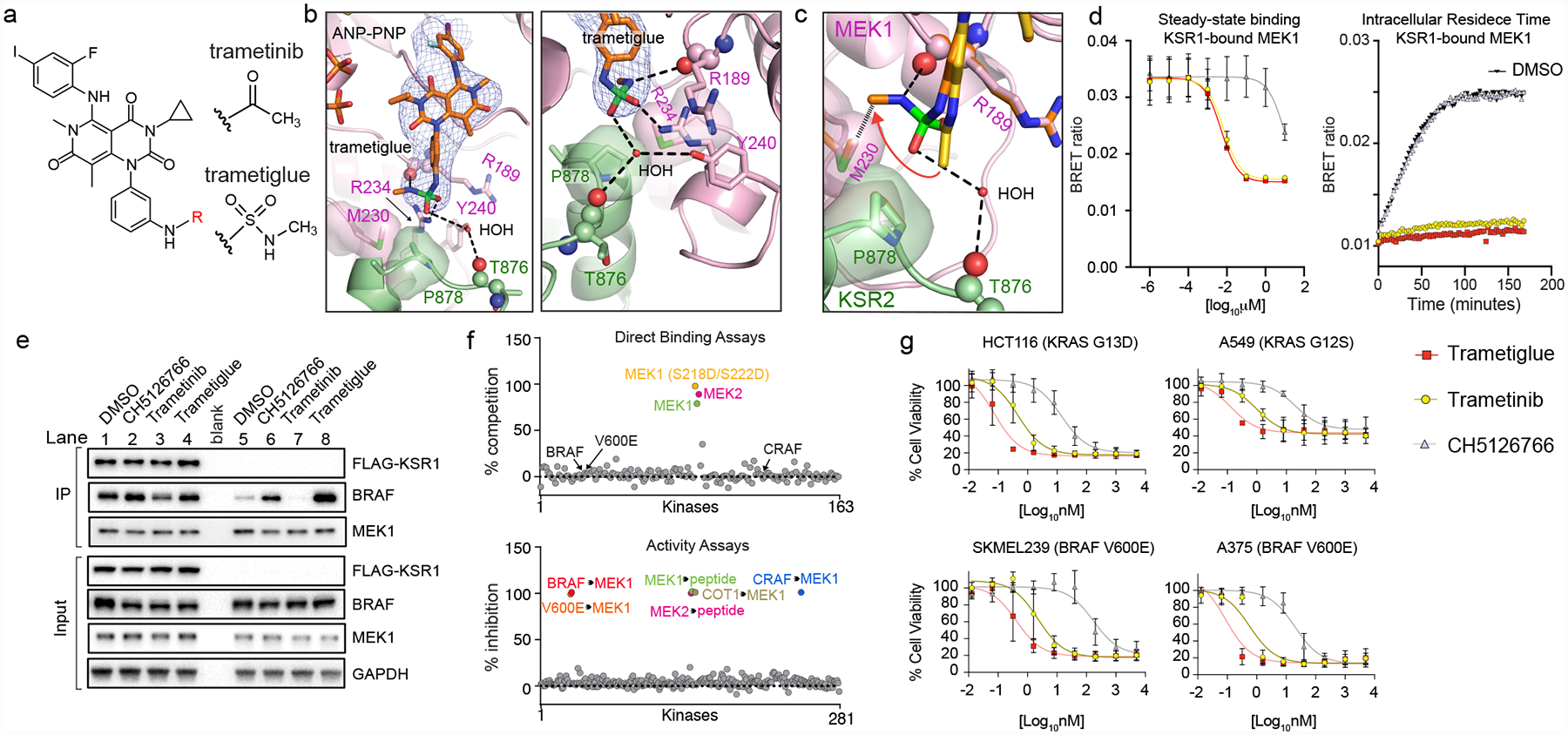
A. Chemical structures of trametinib and trametiglue.
B. X-ray crystal structure of trametiglue bound to KSR2:MEK1:AMP-PNP. Fo-Fc omit electron density map, contoured at 3.0 σ with a 2.0 Å cutoff around ligand. Left panel shows the entire inhibitor binding pocket; right panel highlights contacts around the sulfamide group of trametiglue.
C. Overlay of trametinib (yellow) and trametiglue (orange). The sulfamide group of trametiglue, relative to the acetamide in trametinib, generates unique contacts at the interfacial binding region of KSR-bound MEK. In particular, the -NHSO2NHCH3 module of trametiglue facilitates unique space-filling via M230 and the peptide backbone of R189 in MEK1 and a water-mediated H-bond towards the backbone of T876 in KSR2.
D. Trametiglue retains the strong binding potency and residence time of trametinib on KSR-bound MEK as determined under steady-state conditions (left) and intracellular residence (right panel; all compounds tested at 6.25 nM) formats. Each point and error bars represent the mean and SEM of three independent experiments. Data points for the intracellular residence time experiments represent the average of two technical replicates, each repeated three independent times. Additional data in Supplementary Figure 2.
E. Trametiglue, unlike trametinib but similar to CH5126766, enhances interactions between endogenous BRAF and MEK1. IP/WB of endogenous MEK1 from HCT116 cells. Lanes 1–4 are cells transfected with FLAG-KSR1, and lanes 5–8 are untransfected samples. Cells were treated with DMSO, 200 nM CH5126766, 200 nM trametinib, or 200 nM trametiglue for 1 hour prior to harvesting cells and IPs. Blots are representative of three independent experiments with similar results.
F. In vitro profiling of 1 μM trametiglue demonstrates high selectivity towards MEK1 and MEK2 in direct binding assays (top). Trametiglue also displays high selectivity in a panel of active kinases measured for inhibition of MEK1 and MEK2 substrate phosphorylation or direct MEK1 phosphorylation by the upstream kinases as indicated (bottom). See Source Data Fig. 4.
G. Cell viability dose-responses on K-RAS and BRAF mutant lines. Assays conducted under low-adherence conditions and representative of three independent experiments, each conducted in technical triplicate. Mean and standard deviations in Extended Data Figure 10B.
