Extended Data Figure 1: Summary of ligand bound complexes of KSR1:MEK1 and KSR2:MEK1.
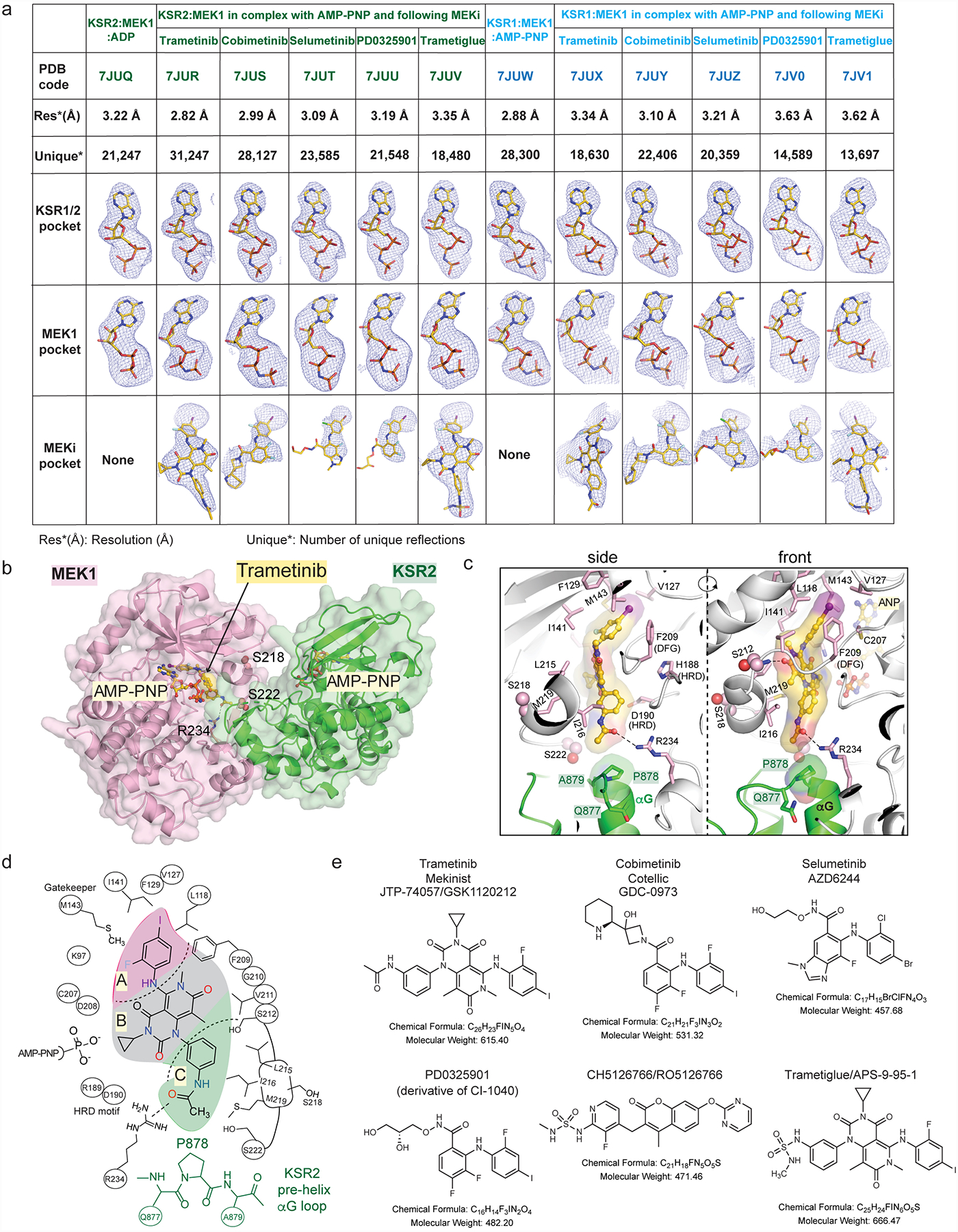
A. Resolution, number of reflections, and ligand omit maps for all described structures. Detailed data collection and refinement statistics are provided in Supplemental Data Table 1. Fo-Fc omit electron density maps are all contoured at 3.0 σ, with a 2.0 Å cutoff, around the ligands and shown as a blue mesh.
B. Trametinib bound to KSR2:MEK1:AMP-PNP.
C. Trametinib contacts include P878 in the pre-helix aG loop of KSR2. Direct contacts of trametinib with MEK1 also highlighted.
D. 2D schematic of the trametinib binding pocket in KSR2:MEK1.
E. 2D structures, formulas, and molecular weights of MEK inhibitors (MEKi) used in this study.
