Scheme 4.
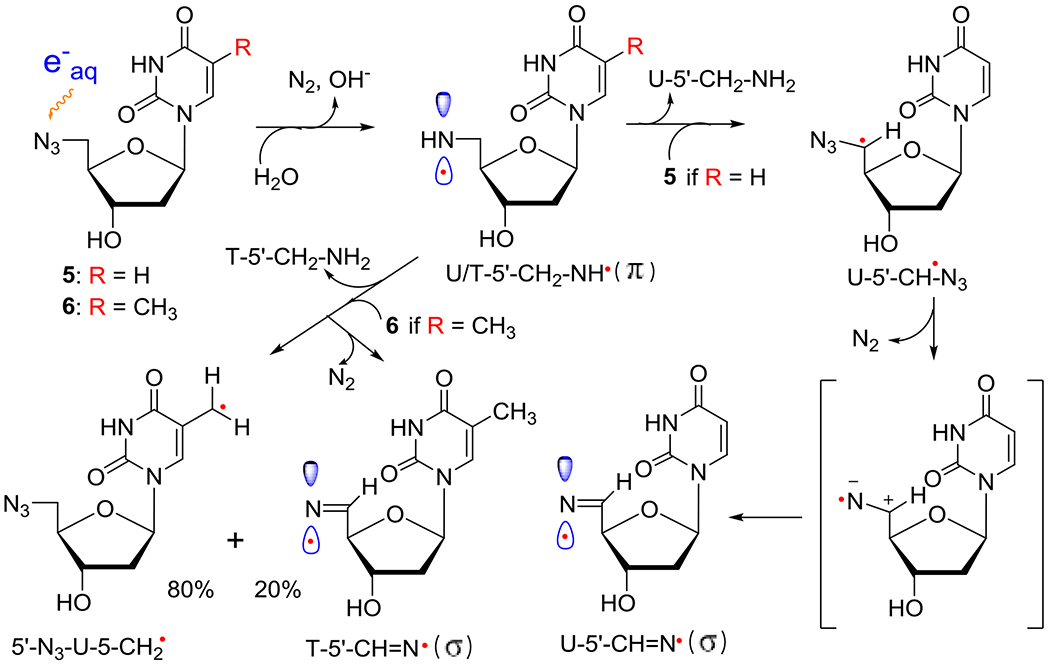
Formation of neutral π-aminyl radical, RNH• from 5′-AZ-2′,5′-ddU (5) and 5′-AZT (6) and its subsequent bimolecular reactions with 5 or 6, respectively. H-atom abstraction from the CH3 group at C5 in 6 by RNH• prevails (ca. 80%) in competition to the H-atom abstraction by the α-azidoalkyl radical, U-5′-CH•-N3, from 5′-CH2 (ca. 20%) in 6 forming the σ-iminyl radical (R=N•). As the allylic radical, U-5-CH2•, is known to undergo either dimerization or addition to the >C=C< double bond,4,33,34 we expect that the allylic radical from 6, 5′-N3-U-5-CH2•, would undergo similar reactions. However, owing to the absence of the CH3 group in 5, H-atom abstraction by the α-azidoalkyl radical, U-5′-CH•-N3, from 5′-CH2 is the only reaction taking place, leading to the facile formation of σ-iminyl radical (R=N•).
