Extended Data Figure 1. Optimization of expression and purification of human K2P4.1 for native ion mobility mass spectrometry studies.
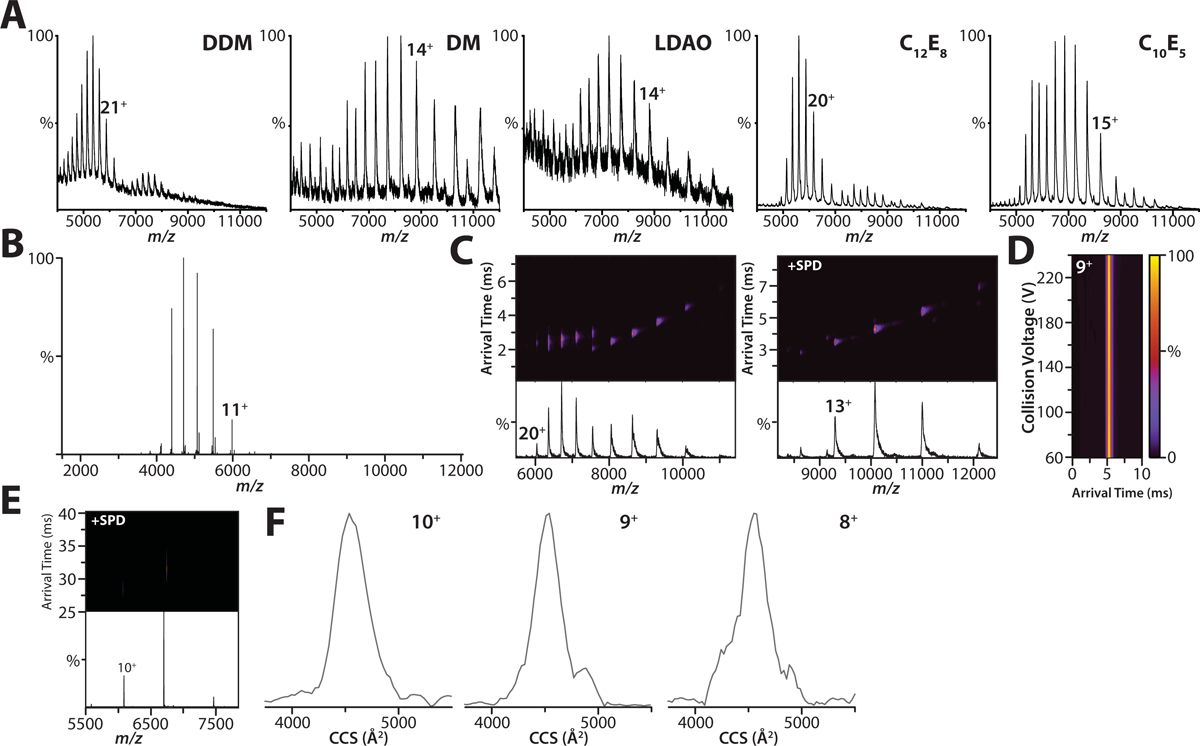
A) Shown are representative mass spectra of the K2P4.1b fusion protein acquired on a Synapt G1 HDMS instrument. Denoted in the inset is the detergent abbreviation: dodecyl maltoside (DDM); decyl maltoside (DM); lauryldimethylamine-N-oxide (LDAO); pentaethylene glycol monodecyl ether (C10E5); and octaethylene glycol monododecyl ether (C12E8). The polyethylene glycol detergents, C10E5 and C12E8, yielded well resolved mass spectra compared to the other detergents screened. B) Membrane proteins were expressed with affinity tags fused to both termini. Shown is the native mass spectrum for K2P4.1b with tags removed by TEV protease in C10E5 acquired on an Orbitrap. C) Native ion mobility mass spectra of the K2P4.1a fusion protein solubilized in (left) 2x CMC C10E5 and with the addition of (right) 50 mM spermidine acquired on Synapt G1. In the absence of charge-reducing molecule, the arrival time distributions are broad for high charge states indicating collisional activation (or partial unfolding) of the protein complex. D) Collision induced unfolding (CIU) plot of arrival time distribution for the 9+ charge state of K2P4.1a with tags removed in C10E5 and spermidine acquired under different collision voltages (10V step size) on a Synapt G1 instrument. The arrival time distribution for the 9+ and other charge states of K2P4.1a remain constant showing that charge reduction aids retention of native-like structure, even at the highest collision voltage (240V). E) Ion mobility mass spectrum for K2P4.1a in C10E5 and spermidine recorded on an Orbitrap UHMR equipped with a REIS and 1.5m drift tube operating in the FT-mode. F) Collision cross section profiles for different charge states of K2P4.1a. The centroid CCS for the 10+, 9+, and 8+ are 4559, 4528, 4547 Å2, respectively. The calculated CCS for PDB 4WFE is 4656 Å2.
