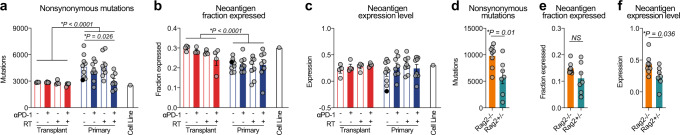
Fig. 2. Evidence of immune editing at the DNA and RNA levels in primary tumors.
a Nonsynonymous mutations in transplant and primary p53/MCA tumors harvested 3 days after specified treatment. The original primary tumor (filled, black) used to generate the cell line (right) from which the transplant tumors were derived is also shown. P < 0.001 for transplant vs primary tumors, P = 0.026 for primary tumors with isotype vs primary tumors with anti-PD-1 and 20 Gy. b Fraction of neoantigenic mutations expressed by RNA sequencing (>5 mutant and >5 WT reads). P < 0.001 for transplant vs primary tumors. c Average expression level of genes with neoantigens in transplant tumors, primary tumors, and the cell line quantified as fragments per kilobase of transcript per million mapped reads (FPKM), upper quartile normalized, log2 transformed. d Nonsynonymous mutations in primary p53/MCA tumors from Rag2−/− and Rag2+/− mice harvested when tumor volume reached 70–150 mm3. P = 0.01 for Rag2−/− vs Rag2+/−. e Fraction of neoantigenic mutations expressed by RNA sequencing (>5 mutant and >5 WT reads). f Average expression level of genes with neoantigens, calculated as in (c). P = 0.036 in Rag2−/− vs Rag2+/−. For a–f, each symbol represents an individual mouse. Mean ± SEM. For a–c transplant tumors: n = 4; primary tumors: n = 8; significance determined by three-way ANOVA with Tukey’s multiple comparisons test. For d–f Rag2−/− tumors: n = 8; Rag2+/− tumors: n = 7; significance determined by unpaired two-tailed t-test. Source data are provided as a Source data file.