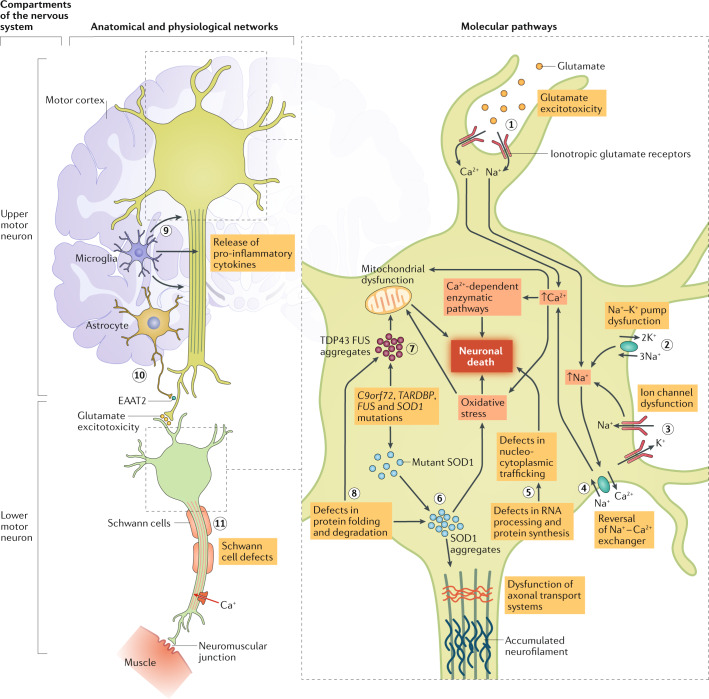
Fig. 1. The pathophysiology of ALS.
Amyotrophic lateral sclerosis (ALS) preferentially involves the descending corticospinal motor neurons that synapse with spinal motor neurons and project to skeletal muscles via the neuromuscular junction. The processes of neurodegeneration in amyotrophic lateral sclerosis involve a complex array of molecular and genetic pathways. Glutamate-induced excitotoxicity can result from the overactivation of ionotropic glutamate receptors that allow excessive influx of Na+ and Ca2+ ions (step 1) and ultimately neurodegeneration through the activation of Ca2+-dependent enzymatic pathways. Glutamate excitotoxicity also generates free radicals, which further contribute to the process of neurodegeneration via oxidative stress. Na+–K+ pump dysfunction (step 2) disrupts the resting membrane potential and leads to secondary effects of altered intracellular Na+ levels. Ion channel dysfunction (step 3) also leads to altered intracellular Na+ levels. Altered Na+ levels result in the reversal of the Na+–Ca2+ exchanger (step 4), thereby increasing the intracellular Ca2+ levels that can cause neuronal toxicity. Defects in RNA processing, RNA metabolism and protein synthesis lead to defects in nucleocytoplasmic trafficking associated with neuronal degeneration (step 5). Mutant SOD1 enzymes increase oxidative stress, induce mitochondrial dysfunction, form intracellular aggregates, and adversely affect neurofilament and axonal transport processes (step 6). Mutations in TARDBP, FUS and C9orf72 can result in the formation of intracellular aggregates of their protein products (step 7), leading to increased oxidative stress, mitochondrial dysfunction, defects in axonal transport and, consequently, in neuronal death. Defects in protein folding and degradation also lead to protein aggregates (step 8). The activation of microglia promotes the secretion of pro-inflammatory cytokines and neurotoxic substances, such as glutamate, which promote neuroinflammation and neuronal death (step 9). Reduced expression and activity of the astrocytic glutamate transporter excitatory amino acid transporter 2 (EAAT2) (step 10) is associated with motor neuron degeneration owing to glutamate toxicity. Accumulation of mutant SOD1 protein in Schwann cells (step 11) are thought to mediate synaptic denervation, which precedes the onset of anterior horn cell degeneration.