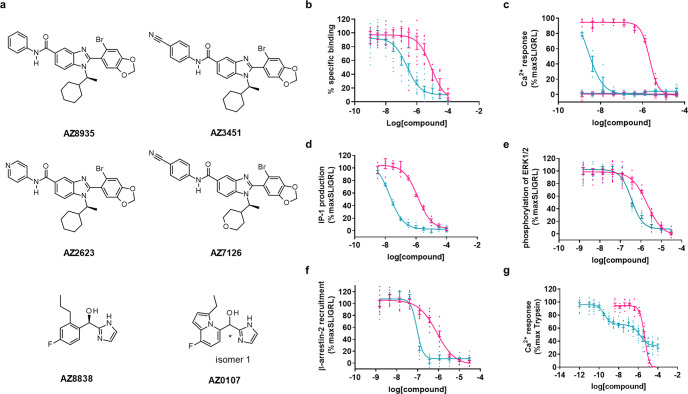
Fig. 1. Pharmacological characterisation of PAR2 antagonists.
a Chemical structures of novel PAR2 antagonists. Benzimidazole antagonist (AZ8935) and optimised analogue structures (AZ3451, AZ2623 and AZ7126). Imidazole antagonist (AZ8838) and representative series analogue (AZ0107). b Binding of both series was evaluated in competition binding experiments against 2f-LIGRLO-(dtpa)Eu in CHO-hPAR2 cells. AZ8838 (magenta) and AZ3451 (cyan) had no effect when tested as agonists (open symbols) and inhibited SLIGRL-NH2 (SLIGRL) activation (closed symbols) of c Ca2+ mobilisation, d IP1 formation, both in 1321N1-hPAR2 cells and e phosphorylation of ERK1/2 and f β-arrestin-2 recruitment, both in U2OS-hPAR2 cells. g Both series also inhibited trypsin activation of PAR2 in Ca2+ mobilisation in 1321N1-hPAR2 cells but interestingly AZ3451 gave a biphasic response whereas AZ8838 was monophasic. Inhibition of trypsin was evaluated in the presence of 1 µM Vorapaxar to suppress any contributions by PAR1 endogenously present in 1321N1 cells. Antagonist responses were calculated as % inhibition of agonist response in the absence of the antagonist. Graphs show representative data of 2 or more experiments, presented as individual data points with error bars denoting s.e.m. The comprehensive pharmacological data are stated in Table 1.