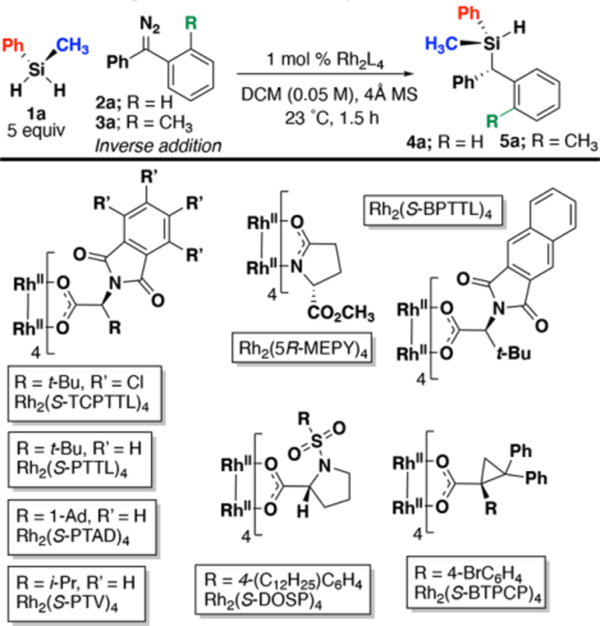
Table 1.
Optimization of donor/donor Si–H insertion
| entry | R | Rh2L4 | % yielda | drb | erc |
|---|---|---|---|---|---|
| 1 | H | Rh2(OAc)4 | 34 | - | 50:50 |
| 2 | H | Rh2(5R-MEPY]4 | <5 | - | ND |
| 3 | H | Rh2(S-BTPCP)4 | <5 | - | ND |
| 4 | H | Rh2(S-DOSP)4 | 65 | - | 55:45 |
| 5 | H | Rh2(R-PTAD)4 | 67 | - | 61:39 |
| 6 | H | Rh2(S-PTTL)4 | 62 | - | 64:36 |
| 7 | H | Rh2(S-BPTTL)4 | 62 | - | 64:36 |
| 8 | H | Rh2(S-PTV)4 | 67 | - | 59:41 |
| 9 | H | Rh2(S-TCPTTL)4 | 76 | - | 76:24 |
| 10 | H | Rh2(S-TCPTTL)4d | 78 | - | 82:18 |
| 11 | CH3 | Rh2(OAc)4 | 45 | 55:45 | 50:50 |
| 12 | CH3 | Rh2(R-PTAD)4 | 72 | 60:40 | ND |
| 13 | CH3 | Rh2(S-DOSP)4 | 75 | 61:39 | ND |
| 14 | CH3 | Rh2(S-TCPTTL)4d | 91 | 93:7 | 93:7 |
| 15e | CH3 | Rh2(S-TCPTTL)4d | 81 | 93:7 | 93:7 |
NMR yield using Ph-TMS as an internal standard.
Determined using 1H NMR Spectroscopy.
Determined using CSP-HPLC analysis of silanol obtained from Pd/C hydrolysis; major diastereomer if relevant.
Toluene used as a solvent.
Diazo added via syringe over five minutes.