Fig. 1 |. NDGA induces disorder at the active site of Stable-5-LOX.
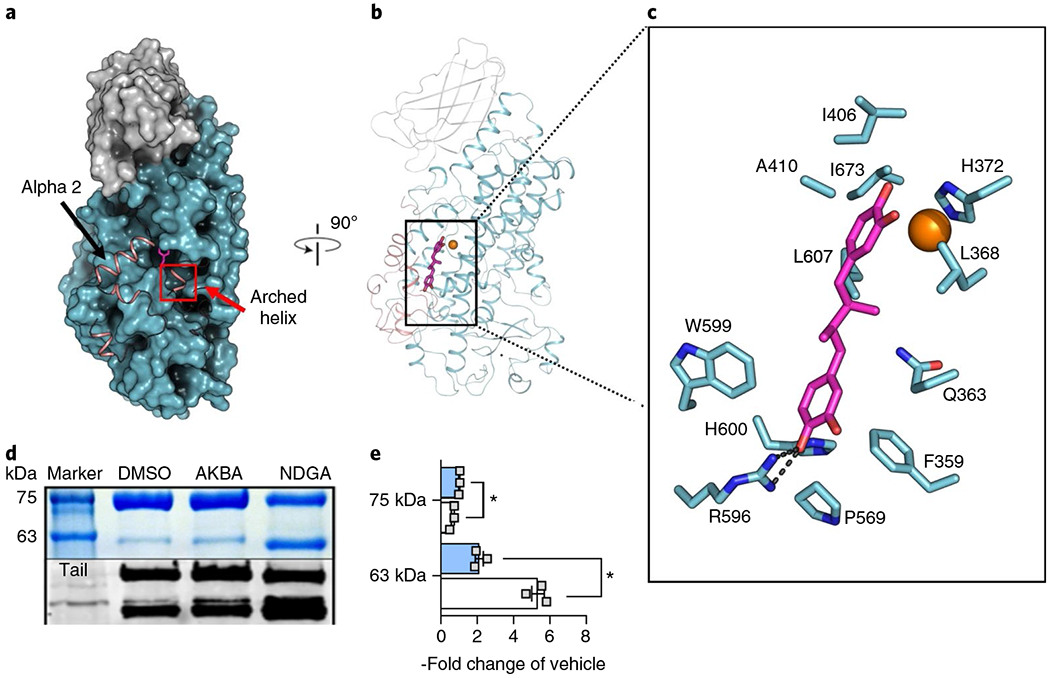
a, A surface rendering of Stable-5-LOX with the amino-terminal domain in gray and catalytic domain in cyan. Electron density is not observed for the polypeptide in the salmon ribbon, modeled from the apo structure (3O8Y). NDGA (magenta stick rendering) is visible in an open cavity. b, The corresponding ribbon diagram rotated 90° with the catalytic Fe as an orange sphere. c, NDGA (C, magenta; O, red) and surrounding amino acids (C, cyan; N, blue; O, red) within 4 Å of the inhibitor, as identified with LigPlot v.2.1 (ref. 49). A single polar contact is provided by R596. d, Proteolysis of Stable-5-LOX in the absence and presence of inhibitors. DMSO as vehicle, AKBA or NDGA were incubated with Stable-5-LOX and the samples were subjected to limited proteolysis using pepsin. The SDS gel (above, see also Supplementary Fig. 3) and western blot (below) of proteolysis products reveal a 63 kD fragment recognized by an antibody to the 5-LOX C-terminus, consistent with cleavage in the region of helix αlpha2. e, Bar graph (+AKBA, blue; + NDGA, white) of band intensity data shown as the fold change of vehicle control from three independent experiments, Student’s paired t-test, *P < 0.05 AKBA versus NDGA.
