Figure 3. P. gingivalis dissociates immune clearance from inflammatory responses in neutrophils.
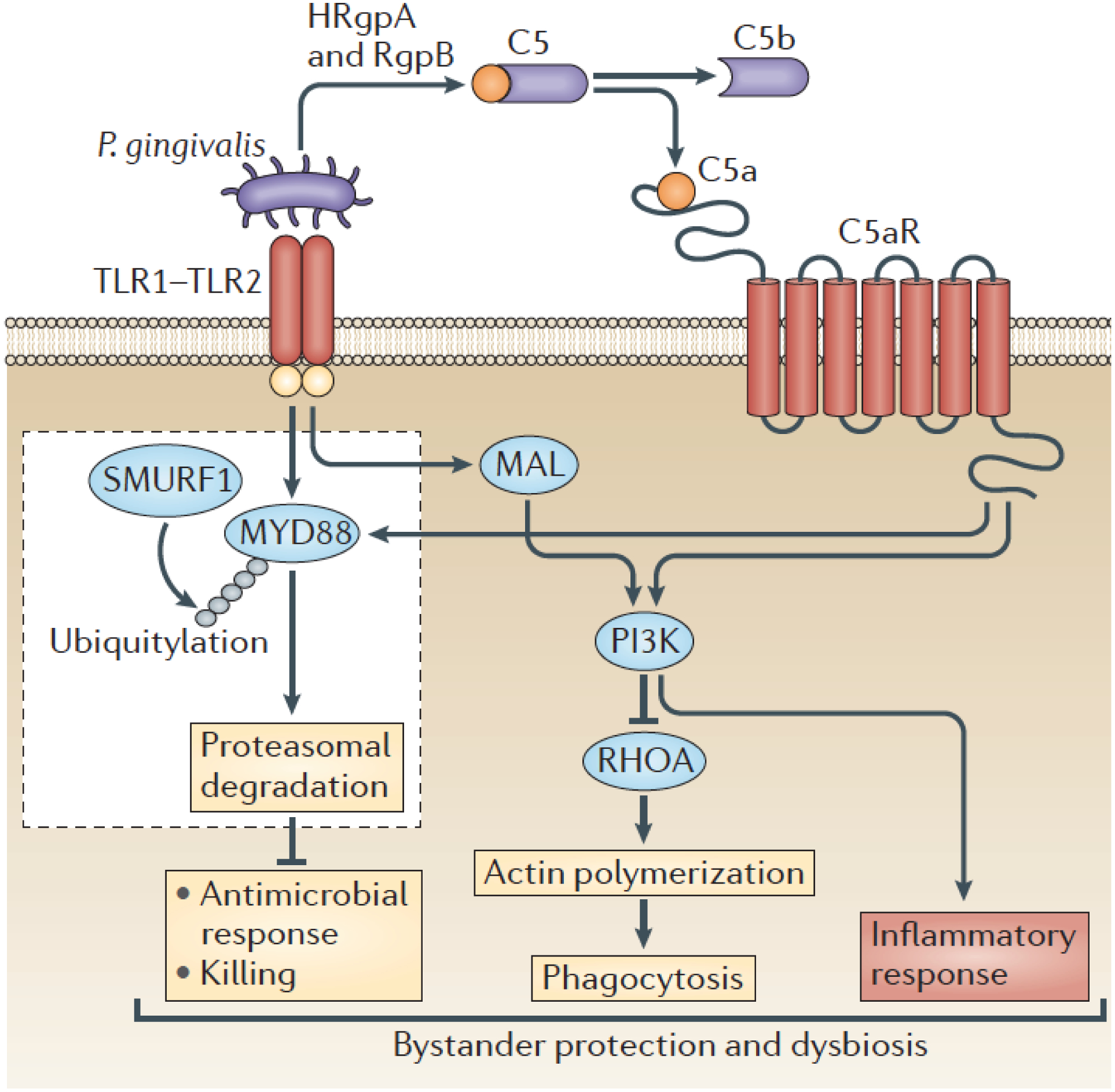
P. gingivalis activates the TLR1-TLR2 heterodimer and C5aR1, the latter through the generation of C5a by its gingipains. Specifically, these enzymes (HRgpA and RgpB) can cleave C5 to release biologically active C5a. The co-activation of TLR2 and C5aR1 by P. gingivalis and the resulting signaling crosstalk leads to the ubiquitylation (via SMURF, an E3 ubiquitin-protein ligase) and proteasomal degradation of the TLR2 adaptor MYD88, thereby blocking a host-protective antimicrobial mechanism. Moreover, the TLR2/C5aR1 crosstalk activates PI3K, which limits phagocytosis through the inhibition of the GTPase RHOA and hence actin polymerization. On the other hand, PI3K stimulates the production of inflammatory cytokines. Contrary to MYD88, another TLR2 adaptor, MYD88-like adaptor protein (MAL), participates in immune subversion by acting upstream of PI3K. These functionally integrated pathways offer ‘bystander’ protection to otherwise susceptible periodontal organisms and enhance polymicrobial dysbiotic inflammation in vivo. From reference [6]. Used by permission.
