Abstract
Skin cancer diagnoses are rising due to increasing ultraviolet ray exposure and an aging population. The complete surgical excision of skin cancer, including a normal tissue, has been the widely performed and determining the adequate safety margin is essential. In this study, we compared the preoperative thickness and width of skin cancer by ultrasonography with the measurements by histopathologic findings.
A total of 211 patients were enrolled in this study and ultrasonography was performed on 30 patients. The width (long and short axis) and thickness of the skin cancers were measured using electronic calipers of ultrasonographic calipers preoperatively and microscope postoperatively.
The skin cancers were basal cell carcinoma (n = 17), squamous cell carcinoma (n = 10), Merkel cell carcinoma (n = 1), mucinous carcinoma (n = 1), and sebaceous carcinoma (n = 1). The mean width (long and short axis) and thickness of the cancers measured by ultrasonography was 1.25 (0.76) cm, 0.96 (0.65) cm, and 0.37 (0.28) cm. The measurements by histopathology was 1.24 (0.84) cm, 0.95 (0.65) cm, and 0.27 (0.24) cm. Kendall's tau-b correlation coefficient between measurements by ultrasonography and histopathology was as follows: long axis, r = 0.733, P < .001; short axis, r = 0.671, P < .001; thickness, r = 0.740, P < .001. Spearman's rank correlation coefficient between measurements by ultrasonography and histopathology was as follows: long axis, r = 0.865, P < .001; short axis, r = 0.829, P < .001; thickness, r = 0.842, P < .001. The difference in mean thickness between the total excised tissue and the skin cancer was 0.29 (0.43) cm (range 0.05–0.40 cm) in basal cell carcinoma and 0.56 (0.58) cm (range 0.05–2.22 cm) in squamous cell carcinoma.
Ultrasonography can accurately measure the width and thickness of skin cancer and predict the safety margins of the wide excision. Preoperative ultrasonography is a good diagnostic tool for surgical planning. Additional studies with larger populations are needed to quantify the range of vertical safety margins.
Keywords: excision margin, pathology, skin cancer, ultrasonography
1. Introduction
Skin cancer diagnoses are rising due to increasing ultraviolet ray exposure and an aging population.[1] The majority of new skin cancer diagnoses are non-melanocytic skin cancers, such as squamous cell carcinoma and basal cell carcinoma.[2] Non-melanocytic skin cancer rarely metastasizes; therefore, a complete surgical excision is the primary treatment for this type of skin cancer. Surgical excision includes the removal of a margin of healthy skin, because the microscopic margin of the cancer often extends beyond its macroscopic margin. The primary objective is to completely excise the cancer, which will prevent recurrence and metastasis. However, excessively wide skin margins should be avoided because they needlessly sacrifice normal tissue. The width (short and long axis) of skin cancer can be visually confirmed; however, the thickness of a lesion can only be visually determined after excision. In addition, the exact range of vertical excision margins for non-melanocytic skin cancer is poorly documented.
Various imaging methods, such as computed tomography, magnetic resonance imaging, and ultrasonography, can be preoperatively performed to accurately characterize skin cancers. Ultrasonography is a noninvasive, radiation-free procedure used in many different medical specialties. Ultrasonography uses the echoes from an ultrasonic pulse that is emitted through a transducer probe to delineate objects or areas of different density and form an image on a screen. Ultrasonography is easily performed and can be used to check the thickness of skin cancer, relationship of skin cancer with surrounding soft and hard tissues (e.g., cartilage and bone), and lymph node metastasis. The examination and histopathologic findings of preoperative biopsies have been the primary method to establish surgical plans for skin cancer. However, preoperative ultrasonography can be used for the objective measurements of skin cancers to determine surgical plans.[3]
Our primary aim in this study was to measure the preoperative thickness and width of skin cancer by ultrasonography and compare these measurements with postoperative histopathologic findings. Our secondary aim was to evaluate the safety margins by measuring the thickness of both excised tissues and their associated skin cancer margins.
2. Materials and methods
A total of 211 patients visited our clinic from January 2009 to April 2020. All patients were diagnosed with one of the following primary malignant skin or soft tissue cancers: squamous cell carcinoma, basal cell carcinoma, mucinous carcinoma, Merkel cell carcinoma, or sebaceous carcinoma. A preoperative ultrasonography was performed on 30 patients. A total of 27 patients were diagnosed in another clinic by a punch biopsy on the suspicious skin lesion, and three patients were diagnosed in our clinic by an excisional biopsy.
Three ultrasonographic scanners were used to obtain B-scan images, and all scans were performed by the same experienced radiologist. Three patients were scanned with an IU22 scanner (Philips, USA) using a linear probe (12 MHz), 15 patients were scanned with a LOGIQ E9 XDclear 2.0 scanner (GE Healthcare, USA) using linear probes (15 and 9 MHz), and 12 patients were scanned with an Aplio i800 scanner (Canon, Japan) using linear probes (18 and 11 MHz).
The width (long and short axis) and thickness of the patients’ skin cancers were measured using electronic calipers. We determined the maximal cancer thickness by measuring the vertical distance between the surface of the skin and the deepest point of the hypoechoic zone. The ultrasonographic measurements of width (long and short axis) of the patients’ skin cancers were performed by applying the ultrasonographic probe in parallel to the long and short axis of the cancer.
All skin cancers were excised and sent to the department of pathology. We performed a gross histological examination of the excised tissues and recorded the findings, which included the width of both the total excised tissue and the skin cancer. The excised tissues were then fixed, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The thickness of the skin cancer was measured under a microscope from the stratum granulosum to the deepest point of the major cancer infiltration. Histopathologists who were not aware of the ultrasonographic findings reported the skin cancer findings, which included the thickness and width.
The thickness and width of the skin cancers that were measured by both ultrasonography and histopathology were entered into a database. Statistical analyses were performed using SPSS software (SPSS Statistics 26, IBM). The ultrasonographic measurements were compared with the histopathologic measurements using Spearman's rank and Kendall's tau-b correlation coefficient.
The study protocol was approved by the Institutional Review Board (IRB, number: 2018-07-027). The patients have provided informed consent for publication of the case. All procedures in the study that involved human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
3. Results
A total of 30 patients (16 males and 14 females) were enrolled in this study. The median age of patients was 71.9 (11.8) years (range, 46–87 years). The skin cancer diagnoses of the enrolled patients were basal cell carcinoma (n = 17), squamous cell carcinoma (n = 10), Merkel cell carcinoma (n = 1), mucinous carcinoma (n = 1), and sebaceous carcinoma (n = 1) (Figs. 1–3). The skin cancers were located on the head and neck (n = 25, 83.3%), extremities (n = 3, 10.3%), and trunk (n = 2, 6.8%) (Table 1). The mean width (long axis) of the cancers measured by ultrasonography and histopathology was 1.25 (0.76) cm and 1.24 (0.84) cm, respectively. The mean width (short axis) of the cancers measured by ultrasonography and histopathology was 0.96 (0.65) cm and 0.95 (0.65) cm, respectively. The mean thickness of the cancers measured by ultrasonography and histopathology was 0.37 (0.28) cm and 0.27 (0.24) cm, respectively (Fig. 4). There was little difference in the mean width (long and short axis) between ultrasonography and histopathology. However, ultrasonography had a higher mean thickness than histopathology. There was a moderate correlation in width between ultrasonography and histopathology in Kendall's tau-b correlation coefficient (long axis, r = 0.733, P < .001; short axis, r = 0.671, P < .001; thickness, r = 0.740, P < .001). There was a strong correlation in width between ultrasonography and histopathology in Spearman's rank correlation coefficient (long axis, r = 0.865, P < .001; short axis, r = 0.829, P < .001; thickness, r = 0.842, P < .001) (Table 2, Fig. 5). A wide excision was performed on all skin cancers. The basal cell carcinomas were excised with a horizontal safety margin range of 0.3 to 1 cm, and the squamous cell carcinomas were excised with a horizontal safety margin range of 0.5 to 1 cm. The Merkel cell carcinoma was excised with a horizontal safety margin of 2 cm. The majority of the excisions included the subcutaneous fat layer in the vertical range. In some cases, the superficial musculoaponeurotic system, platysma muscle, superficial fascia, and deep fascia were excised, and superficial muscle was partially removed (Table 3). If the frozen biopsy that was performed during the operation showed marginal cancer infiltration, an additional extensive excision was performed on the affected area. The operations were completed when we could no longer identify cancer infiltration of the margin. The difference in mean thickness between the total excised tissue and the skin cancer was 0.29 (0.43) cm (range 0.05–0.40 cm) in basal cell carcinoma and 0.56 (0.58) cm (range 0.05–2.22 cm) in squamous cell carcinoma. Eight patients were on follow-up for 1 to 2 years, and 21 patients were on follow-up for 1 year. No patients had a recurrence of their skin cancer during the follow-up period.
Figure 1.
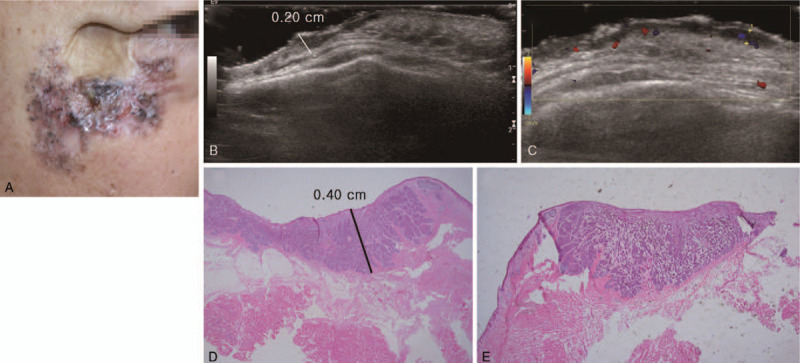
A 65-year-old male patient with basal cell carcinoma on the right cheek and lower eyelid. The patient received a split thickness skin graft on the right cheek 30 years ago due to trauma. (A) Clinical photography. Irregular margined skin lesion with multiple pigmentation and crater-like appearance on the right cheek and lower eyelid. (B) Ultrasonography longitudinal scan. (C) Ultrasonography transverse scan. 4.0 × 3.0 × 0.2 cm sized hypoechoic lesion along the skin and subcutaneous fat layer. (D and E) Histopathologic image. The thickness of basal cell carcinoma was 0.40 cm (hematoxylin and eosin stain, magnification 1.25×).
Figure 3.
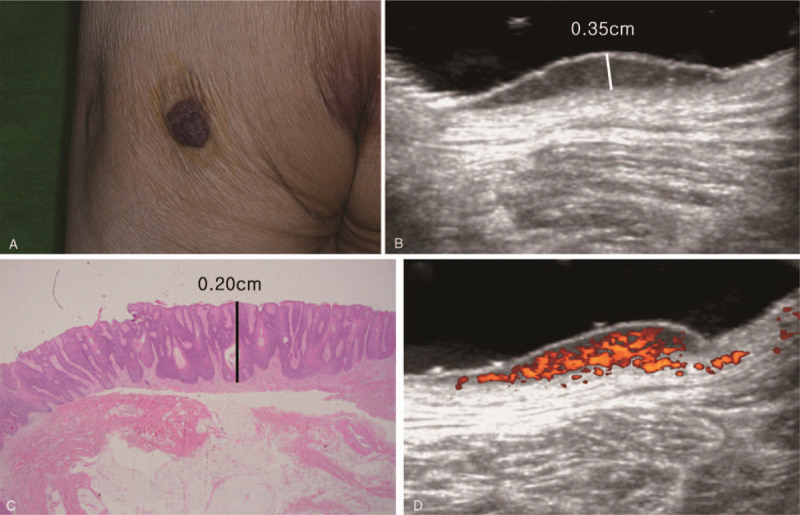
An 83-year-old male patient with squamous cell carcinoma in situ on the left buttock. (A) Clinical photography. Brown pigmented, elevated skin lesion with irregular margin on the left buttock. (B) Ultrasonography longitudinal scan. 2.30 × 2.17 × 0.35 cm sized protruding hypoechoic lesion in the skin layer was observed. (C) Histopathologic image. The thickness of squamous cell carcinoma was 0.20 cm (hematoxylin and eosin stain, magnification 1.25×). (D) Ultrasonography transverse scan. Hypervascularized B-scan image was observed with color doppler.
Table 1.
Patient demographics (n = 30).
| Age | 71.9 (11.8) (46–87) |
| Sex | |
| Male | 16 (53.3%) |
| Female | 14 (46.6%) |
| Diagnosis | |
| Basal cell carcinoma | 17 (56.6%) |
| Squamous cell carcinoma | 10 (33.3%) |
| Merckel cell carcinoma | 1 (3.3%) |
| Mucinous carcinoma | 1 (3.3%) |
| Sebaceous carcinoma | 1 (3.3% |
| Location | |
| Head and neck | 25 (83.3%) |
| Cheek | 9 (30.0%) |
| Nose | 8 (26.6%) |
| Forehead | 2 (6.6%) |
| Neck | 2 (6.6%) |
| Temple | 1 (3.3%) |
| Scalp | 1 (3.3%) |
| Mastoid area | 1 (3.3%) |
| Upper eyelid | 1 (3.3%) |
| Extremities | 3 (10.0%) |
| Index finger | 1 (3.3%) |
| Lower leg | 1 (3.3%) |
| Sole | 1 (3.3%) |
| Trunk | 2 (6.6%) |
| Suprapubic area | 1 (3.3%) |
| Buttock | 1 (3.3%) |
Figure 4.
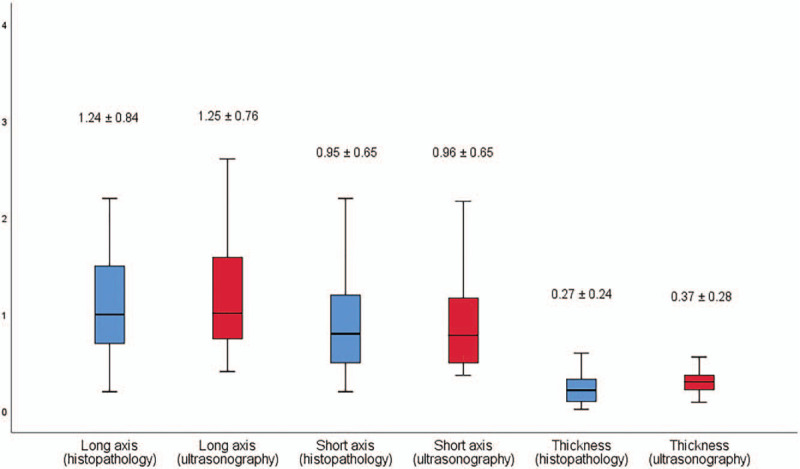
Width (long and short axis) and thickness of skin cancers measured by high-frequency ultrasonography and histopathology.
Table 2.
Width (long and short axis) and thickness of skin cancers measured by high-frequency ultrasonography and histopathology, and the correlation coefficient between measurements of skin cancers by ultrasonography and histopathology.
| Mean (SD) (ultrasonography, cm) | Mean (SD) (histopathology, cm) | Kendall's tau-b correlation coefficient | Spearman's rank correlation coefficient | P | |
| Width (long axis) | 1.25 (0.76) | 1.24 (0.84) | 0.733 | 0.865 | <.001 |
| Width (short axis) | 0.96 (0.65) | 0.95 (0.65) | 0.671 | 0.829 | <.001 |
| Thickness | 0.37 (0.28) | 0.27 (0.24) | 0.740 | 0.842 | <.001 |
Figure 5.
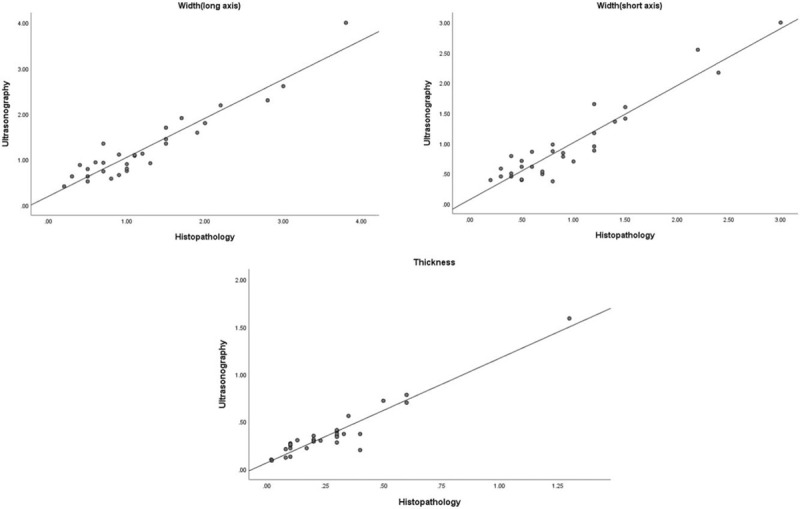
Comparison between measurements (width of long and short axis, thickness) of skin cancers by high-frequency ultrasonography and histopathology.
Table 3.
Characteristics of skin cancers and safety margins.
| No | Sex | Age | Diagnosis | Location | Horizontal margin (cm) | Vertical margin | Follow up (month) | Recurrence |
| 1 | F | 81 | Merkel cell carcinoma | Cheek | 2 | Subcutaneous fat layer | 7.2 | – |
| 2 | M | 83 | Basal cell carcinoma | Cheek | 1 | Subcutaneous fat layer | 3.1 | – |
| 3 | M | 86 | Squamous cell carcinoma | Forehead | 1.5 | Subcutaneous fat layer | 15.0 | – |
| 4 | M | 64 | Basal cell carcinoma | Cheek | 1, 0.5 (lid cheek junction) | Subcutaneous fat (positive deep margin in frozen biopsy) −> Superficial muscle (negative deep margin) | 4.6 | – |
| 5 | F | 61 | Basal cell carcinoma | Neck | 1 | Subcutaneous fat layer | 8.9 | – |
| 6 | F | 87 | Basal cell carcinoma | Scalp | 0.5 | Subcutaneous fat layer | 3.1 | – |
| 7 | M | 77 | Basal cell carcinoma | Nose | 0.5 | Subcutaneous fat layer | 5.1 | – |
| 8 | F | 46 | Basal cell carcinoma | Cheek | 0.5 | Subcutaneous fat layer | 24.0 | – |
| 9 | M | 61 | Basal cell carcinoma | Nose | 0.5 | Subcutaneous fat layer | 23.2 | – |
| 10 | M | 66 | Basal cell carcinoma | Nose | 0.4 | Subcutaneous fat layer | 12.6 | |
| 11 | F | 65 | Basal cell carcinoma | Nose | 0.3 | Subcutaneous fat layer | 20.2 | – |
| 12 | M | 58 | Basal cell carcinoma | Temple | 0.5 | Subcutaneous fat layer | 5.7 | – |
| 13 | M | 84 | Basal cell carcinoma | Cheek | 0.3 | Subcutaneous fat layer | 17.5 | – |
| 14 | F | 79 | Basal cell carcinoma | Nose | 0.4 | Subcutaneous fat layer | 11.5 | – |
| 15 | F | 84 | Squamous cell carcinoma | Cheek | 1 | Subcutaneous fat layer | 13.3 | – |
| 16 | M | 83 | Squamous cell carcinoma in situ | Buttock | 1 | Subcutaneous fat layer | 2.5 | – |
| 17 | M | 69 | Basal cell carcinoma | Cheek | 0.5 | Subcutaneous fat layer | 12.3 | – |
| 18 | M | 46 | Squamous cell carcinoma in situ | Sole | 0.5 | Subcutaneous fat layer | 11.9 | – |
| 19 | F | 80 | Squamous cell carcinoma in situ | Nose | 0.5 | Superficial muscle | 8.3 | – |
| 20 | F | 78 | Squamous cell carcinoma | Nose | 0.4 | Subcutaneous fat layer | 8.6 | – |
| 22 | M | 78 | Squamous cell carcinoma | Cheek | 1 | Superficial muscle | 10.6 | – |
| 21 | F | 79 | Basal cell carcinoma | Forehead | 0.5 | Subcutaneous fat layer | 3.6 | – |
| 23 | M | 77 | Basal cell carcinoma | Nose | 0.5 | Subcutaneous fat layer | 10.0 | – |
| 24 | M | 73 | Squamous cell carcinoma in situ | Index finger | 1 | Deep fascia | 5.9 | – |
| 25 | F | 59 | Squamous cell carcinoma in situ | Suprapubic area | 1 | Superficial fascia | 6.9 | – |
| 26 | F | 77 | Squamous cell carcinoma in situ | Lower leg | 1 | Superficial fascia | 5.6 | – |
| 27 | F | 84 | Basal cell carcinoma | Neck | 0.5 | Plastysma | 3.5 | – |
| 28 | M | 67 | Mucinous carcinoma | Cheek | 1 | Superficial musculoaponeurotic system (SMAS) | 3.1 | – |
| 29 | M | 55 | Basal cell carcinoma | Mastoid area | 1 | Subcutaneous fat layer | 2.6 | – |
| 30 | F | 87 | Sebaceous carcinoma | Upper eyelid | 0.5 | Full thickness eyelid | 2.0 | – |
Figure 2.
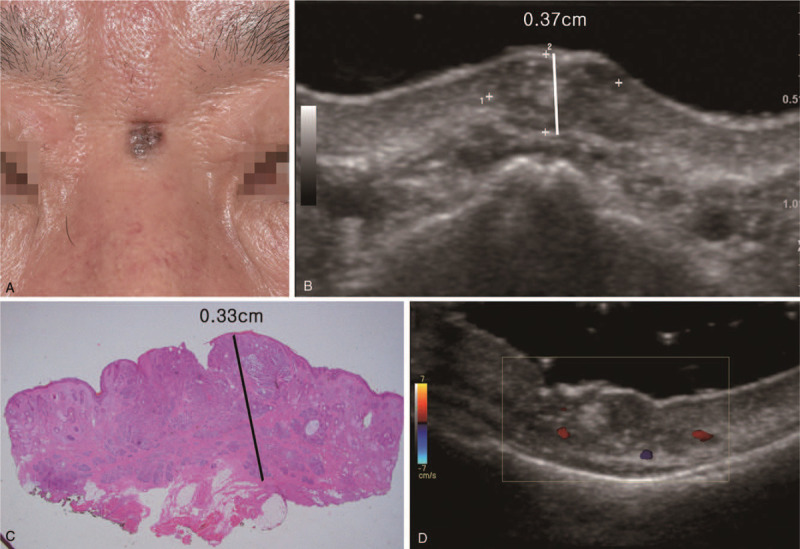
A 61-year-old male patient with basal cell carcinoma on the nasal root. (A) Clinical photography. Gray pigmented, scaled skin lesion with irregular margin on the root of the nose. (B) Ultrasonography longitudinal scan. 0.75 × 0.50 × 0.37 cm sized lobulated mixed echoic lesion in the skin and subcutaneous fat layer. (C) Histopathologic image. The thickness of basal cell carcinoma was 0.33 cm (hematoxylin and eosin stain, magnification 1.25×). (D) Ultrasonography transverse scan.
4. Discussion
The origins of skin and soft tissue cancers are divided into epithelium, appendage, and mesenchium; the most common of these are the cancers that originate in the epithelium, including basal cell carcinoma and squamous cell carcinoma. Skin and soft tissue cancers are relatively easy to detect early because they occur on the skin, which makes them noticeable. The suspected lesion is confirmed histologically by a punch, incisional, or excisional biopsy. In addition to the biopsy, various imaging modalities, such as computed tomography, magnetic resonance imaging, positron emission tomography, and ultrasonography are performed; ultrasonography is the most widely performed.
Ultrasonography is repeatable, noninvasive, painless, and free from the complications found in the other imaging methods, such as adverse reactions to contrast dye and radiation. Ultrasonography can quickly identify the thickness of skin, depth of fat tissue, and fascial integrity, and important structures in a patient, such as arteries, veins, and nerves, can be preoperatively identified to reduce postoperative morbidity. In addition, ultrasonography can detect the underlying cartilage structure in high risk areas, such as the nose and ear, and examine regional lymph nodes for metastasis.[3–5] This information can reduce the unnecessary removal of cancer-free structures and help to develop an appropriate surgical plan.
Basal cell carcinoma is divided into nodular, superficial, and aggressive subtypes. Ultrasonography can be used as a sensitive screening method to discriminate aggressive subtypes.[6] In addition, ultrasonography can be used to screen for the recurrence of skin cancer. The early detection of skin cancer recurrence by visual inspection is difficult when flap coverage is performed after a wide excision; the flaps and postoperative scar cover the excision area. Ultrasonography can be used to accurately detect the presence of pathologic findings that may not be visible, including lymph node recurrence, and ultrasonography images taken over a specific time period can be compared.[7] We performed ultrasonography in our patients every 3 to 6 months after surgery to screen for the recurrence of skin cancer.
The accurate evaluation of skin lesions by ultrasonography requires expensive high-frequency methodology and well-trained technicians. When the ultrasonography probe is large, adequate access to high-risk areas for skin cancer, such as the eyelid, nose, and ear, may be difficult.[3] Similarly, a determination of the exact outline of skin cancer in areas with irregular extensions can be difficult. Ultrasonographic evaluation in areas with scars due to previous biopsy or curettage, or where inflammation exists around the cancer may be difficult; as a result, the size of the cancer may be overestimated.[8,9] The overall cancer measurements by ultrasonography in our study were overestimated when compared to those by histopathology. The ultrasonographic measurements could have been overestimated because inflammation around the cancer lesion makes it difficult to accurately determine the skin cancer margin. It is difficult to measure the skin cancer lesions at the exact same point due to their irregular margins.[10,11]
In the study about tissue shrinkage after excision of basal cell carcinoma, it has been shown that the long dimension of tissue specimens containing skin cancer have shrunk by only 11% after excision, formalin fixation, and histological processing.[12] In addition, authors excised the skin cancer with a sharp blade, thus tissue contraction would have been less than if resected with electrocautery. In the event that this causes a bias, since the skin cancer was excised widely with safety margin of at least 5 mm or more, this would not have been problematic as the skin cancer would still be completely removed.
Various methods are used to treat skin cancer, including surgical excision, laser therapy, chemotherapy, radiation therapy, and immunotherapy. The complete surgical excision of skin cancer, including a normal tissue safety margin, has been the most widely performed and successful method. The optimal goal of skin cancer treatment is resection of the cancer with a maximum preservation of the surrounding healthy tissue and no cancer recurrence. Since the degree of cancer invasion cannot be confirmed with a visual observation, a wide excision that includes the horizontal and vertical safety margins is performed. However, the horizontal and vertical safety margins vary depending on the type of skin cancer. Recent retrospective histopathologic studies have supported reductions in the horizontal safety margin.[13–16] The horizontal safety margins that are recommended for particular cancer types are as follows: basal cell carcinoma, low risk 4 mm, high risk 5 to 10 mm; squamous cell carcinoma, low risk 4 to 6 mm, high risk 10 mm; and Merkel cell carcinoma, 10 to 20 mm.[17–19] The vertical safety margin should be determined by taking into account the anatomic barriers, such as the fat layer, superficial and deep fascia, perichondrium, and periosteum. In general, at least one layer of anatomic barrier should be excised. For example, the deep fascia of skeletal muscle is considered the anatomic barrier for cancers that are limited to the fat layer and should be resected along with the cancer. Histopathologic studies have quantified the range of the horizontal safety margin; however, a quantified range for the vertical safety margin has not been established. The basal cell carcinomas in our patients were excised with an average vertical safety margin of 0.29 (0.43) cm (range 0.05–0.40 cm). The squamous cell carcinomas in our patients were excised with an average vertical safety margin of 0.56 (0.58) cm (0.05–2.22 cm). There was no recurrence of the skin cancers in our patients after the wide excisions. However, we must examine the recurrence rate of skin cancer in a much larger study population in order to accurately quantify the range of the vertical safety margin.
Previous studies have reported a strong correlation between histologic and ultrasonographic thickness for melanoma, basal cell carcinoma, and non-malignant nevi.[6,9,20,21] The correlation between histologic and ultrasonographic thickness in this study was strong, even though we compared live tissue (ultrasonographic findings) with dehydrated tissue (histopathologic findings). Our results reveal that ultrasonography is a good diagnostic modality to evaluate the extent and thickness of skin cancer before surgery.
5. Conclusion
We observed a good correlation between preoperative ultrasonography and postoperative histopathology in this study. Ultrasonography can accurately measure the width and thickness of skin cancer and predict the horizontal and vertical safety margins of the wide excision. Preoperative ultrasonography is a good diagnostic tool for surgical planning. Additional studies with larger patient populations are needed to quantify the range of vertical safety margins for each skin cancer type and area affected.
Author contributions
Conceptualization: Hyeonjo Kim, Seonghwan Kim, In Suck Suh
Data curation: Hyeonjo Kim, Juho Lee, Seho Shin.
Formal analysis: Hyeonjo Kim.
Investigation: Seongjoo Lee, Jaehyun Kim.
Methodology: Hyeonjo Kim, In Suck Suh.
Resources: Huiying Xu, Ik Yang.
Software: Hyeonjo Kim.
Supervision: In Suck Suh.
Writing – original draft: Hyeonjo Kim.
Writing – review & editing: Hyeonjo Kim, In Suck Suh.
Footnotes
Abbreviation: None.
How to cite this article: Kim HJ, Lee SJ, Lee JH, Shin SH, Xu H, Yang I, Kim JH, Kim SH, Suh IS. Usefulness of ultrasonography in determining the surgical excision margin in non-melanocytic skin cancer: A comparative analysis of preoperative ultrasonography and postoperative histopathology. Medicine. 2020;99:51(e23789).
The authors have no conflict of interest to disclose.
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- [1].Zaynoun S, Ali L-A, Shaib J, et al. The relationship of sun exposure and solar elastosis to basal cell carcinoma. J Am Acad Dermatol 1985;12:522–5. [DOI] [PubMed] [Google Scholar]
- [2].Zink A. Non-melanoma skin cancer: pathogenesis, prevalence and prevention. Hautarzt 2017;68:919–28. [DOI] [PubMed] [Google Scholar]
- [3].Bobadilla F, Wortsman X, Muñoz C, et al. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging 2008;8:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bard RL. High-frequency ultrasound examination in the diagnosis of skin cancer. Dermatol Clin 2017;35:505–11. [DOI] [PubMed] [Google Scholar]
- [5].Zeitouni NC, Rohrbach DJ, Aksahin M, et al. Preoperative ultrasound and photoacoustic imaging of nonmelanoma skin cancers. Dermatol Surg 2015;41:525–8. [DOI] [PubMed] [Google Scholar]
- [6].Nassiri-Kashani M, Sadr B, Fanian F, et al. Pre-operative assessment of basal cell carcinoma dimensions using high frequency ultrasonography and its correlation with histopathology. Skin Res Technol 2013;19:e132–138. [DOI] [PubMed] [Google Scholar]
- [7].Catalano O, Roldán FA, Varelli C, et al. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound 2019;22:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].MacFarlane D, Shah K, Wysong A, et al. The role of imaging in the management of patients with nonmelanoma skin cancer: diagnostic modalities and applications. J Am Acad Dermatol 2017;76:579–88. [DOI] [PubMed] [Google Scholar]
- [9].Lassau N, Spatz A, Avril MF, et al. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics 1997;17:1559–65. [DOI] [PubMed] [Google Scholar]
- [10].Crisan M, Crisan D, Sannino G, et al. Ultrasonographic staging of cutaneous malignant tumors: an ultrasonographic depth index. Arch Dermatol Res 2013;305:305–13. [DOI] [PubMed] [Google Scholar]
- [11].Jasaitiene D, Valiukeviciene S, Linkeviciute G, et al. Principles of high-frequency ultrasonography for investigation of skin pathology. J Eur Acad Dermatol Venereol 2011;25:375–82. [DOI] [PubMed] [Google Scholar]
- [12].Blasdale C, Charlton FG, Weatherhead SC, et al. Effect of tissue shrinkage on histological tumour-free margin after excision of basal cell carcinoma. Br J Dermatol 2010;162:607–10. [DOI] [PubMed] [Google Scholar]
- [13].Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg 2003;112:57–63. [DOI] [PubMed] [Google Scholar]
- [14].Schell AE, Russell MA, Park SS. Suggested excisional margins for cutaneous malignant lesions based on Mohs micrographic surgery. JAMA Facial Plast Surg 2013;15:337–43. [DOI] [PubMed] [Google Scholar]
- [15].Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241–8. [DOI] [PubMed] [Google Scholar]
- [16].Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg 2010;126:1222–31. [DOI] [PubMed] [Google Scholar]
- [17]. National Comprehensive Cancer Network. Basal Cell Skin Cancer (Version 1. 2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf [accessed October 24, 2019]. [Google Scholar]
- [18]. National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 1. 2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf [accessed October 2, 2019]. [Google Scholar]
- [19]. National Comprehensive Cancer Network. Merkel Cell Carcinoma (Version 2. 2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf [accessed October 2, 2019]. [Google Scholar]
- [20].Guitera P, Li LX, Crotty K, et al. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br J Dermatol 2008;159:364–9. [DOI] [PubMed] [Google Scholar]
- [21].Hayashi K, Koga H, Uhara H, et al. High-frequency 30-MHz sonography in preoperative assessment of tumor thickness of primary melanoma: usefulness in determination of surgical margin and indication for sentinel lymph node biopsy. Int J Clin Oncol 2009;14:426–30. [DOI] [PubMed] [Google Scholar]


