Figure 1. Metabolic Regulation of α-Ketoglutarate-Dependent Dioxygenases.
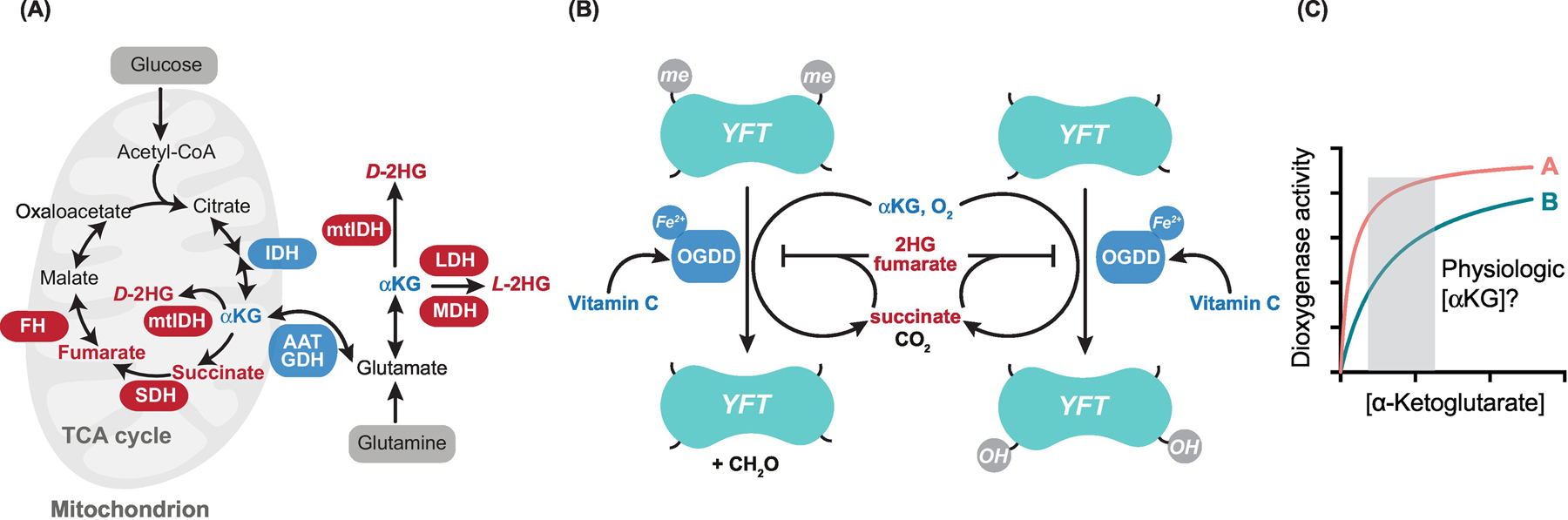
(A) Schematic of key pathways involved in synthesis and break down of α-ketoglutarate (αKG), 2-hydroxyglutarate (2HG), fumarate, and succinate. Enzymes directly involved in αKG metabolism are shown in blue, those involved in 2HG, fumarate, and succinate metabolism are shown in red. (B) Generalized schematic of αKG-dependent dioxygenase (also known as 2-oxoglutarate dependent dioxygenase or OGDD) action on your favorite target (YFT). Dioxygenases catalyze net demethylation or hydroxylation reactions using αKG and molecular oxygen as cosubstrates and producing succinate as a by-product. Vitamin C, oxygen, and αKG can promote dioxygenase activity, whereas succinate, fumarate, and 2HG have been shown to suppress their activity. (C) Enzymatic assays provide potential insights into metabolic regulation of dioxygenase catalytic activity. In this example, dioxygenase B is expected to be sensitive to physiological fluctuations in αKG concentrations, whereas dioxygenase A will be less sensitive. However, it remains unclear what true physiological αKG concentrations are, given that dioxygenases may be sensitive to compartmentalized metabolite pools. Abbreviations: FH, Fumarate hydratase; IDH, isocitrate dehydrogenase; mtIDH, IDH mutations; SDH, succinate dehydrogenase; TCA, tricarboxylic acid.
