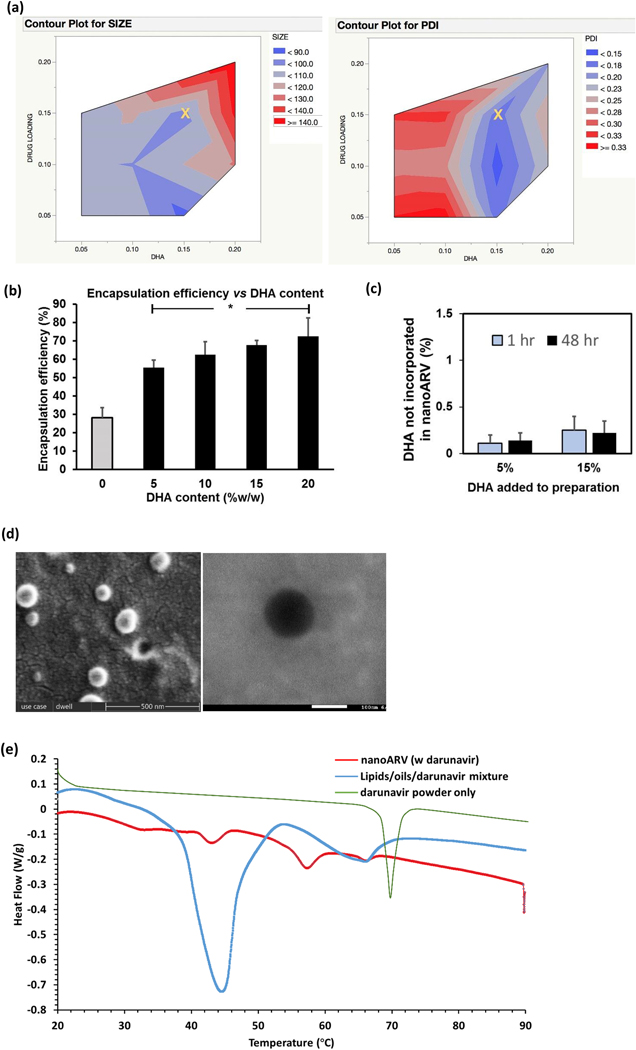
Figure 2.
Physical properties of nanoARVs. (A) Impact of DHA content and drug loading on nanocarrier diameter (left) and polydispersity index (PDI) of nanocarriers (right).The yellow X in each contour graph marks the amounts of DHA and darunavir (15% DHA; 15% intended darunavir payload) most typically used to prepare nanoARVs in the subsequent experiments.(B) Impact of DHA content on darunavir encapsulation efficiency in nanoARV.(C) Quantification of the amount of DHA not encapsulated in nanoARVs (5 or 15% w/w DHA added in preparation. NanoARVs stored at 4 °C). (D) Representative images of SEM (left) and TEM (right) of Tf-nanoARV (15% DHA, 12% darunavir, Tf:lipids=1:10) are shown. (E) Differential scanning calorimetry thermogram of 15%DHA-nanoARVs (12% darunavir) and comparison with darunavir powder and lipids/drug mixture (the first heating run was used for nanoARVs and mixture; second run for darunavir powder). Means+SD (N=3) are shown. *P<0.01 vs no DHA