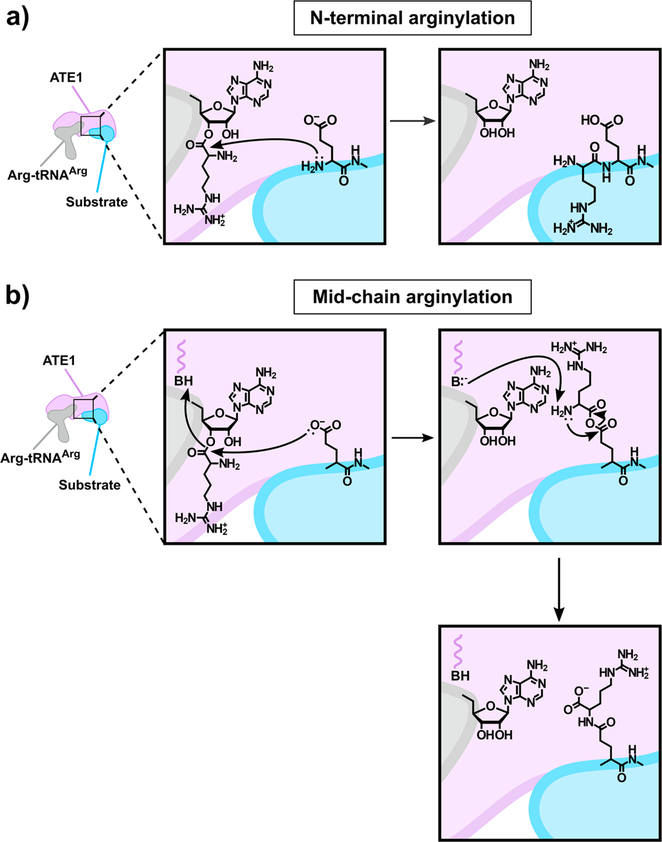
Figure 4.
Cartoon depiction of the proposed mechanism of ATE1-catalyzed arginylation. (a) The canonical ATE1-catalyzed N-terminal arginylation reaction involves the transfer of Arg from the aminoacylated Arg-tRNAArg to the N terminus of the acceptor substrate to form a peptide bond in a nonribosomal manner. The inferred mechanism for this process involves nucleophilic attack of the 3′-acyl group by the N-terminal amino group, with subsequent release of the unmodified tRNAArg and the arginylated substrate. (b) An alternative ATE1-catalyzed reaction involves the transfer of Arg from the aminoacylated Arg-tRNAArg to the internal Asp/Glu side chain of the acceptor substrate to form an isopeptide bond in a nonribosomal manner. The postulated mechanism for this process involves the attack of the Arg-tRNAArg 3′-acyl group by the Asp/Glu carboxylate side chain, proton transfer at an active-site catalytic acid, release of the 3′-OH of the tRNA, and the formation of an unstable anhydride-like intermediate. The active-site conjugate base could then deprotonate the Arg α amine, attacking the anhydride carbonyl internally, releasing the Arg α carboxylate, and forming the isopeptide bond. In both panels, ATE1 is represented as the lavender shape, tRNAArg is represented as the gray shape, and the acceptor substrate protein is represented as the blue shape.