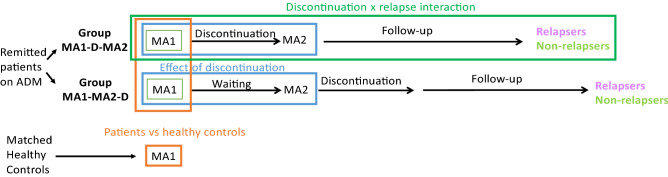
Figure 1.
Study Design: Remitted patients on antidepressant medication (ADM) and matched healthy controls were included in the study and assessed during main assessment 1 (MA1). Patients were randomised to groups MA1-D-MA2 or MA1-MA2-D, where the name indicates the order of events and "D" indicates discontinuation. In group MA1-D-MA2, they underwent MA1, then discontinued their medication followed by main assessment 2 (MA2). In group MA1-MA2-D, they underwent MA1 and MA2 first and only discontinued thereafter. After discontinuation, all patients were followed up for six months to ascertain relapses. Comparison of patient and control groups at MA1 was used to examine the remitted medicated state cross-sectionally (orange). Data collected at MA1 from all patients who subsequently completed the study as relapsers or non-relapsers, i.e. relapsers and non-relapsers from both groups combined were examined to examine associations with relapse. The interaction between time point (MA1 and MA2) with group (MA1-D-MA2 and MA1-MA2-D) examined the impact of discontinuation (blue). Finally, the interaction between time point (MA1 and MA2) and relapse in group MA1-D-MA2 was used to examine changes due to discontinuation that led to relapse or resilience (dark green).