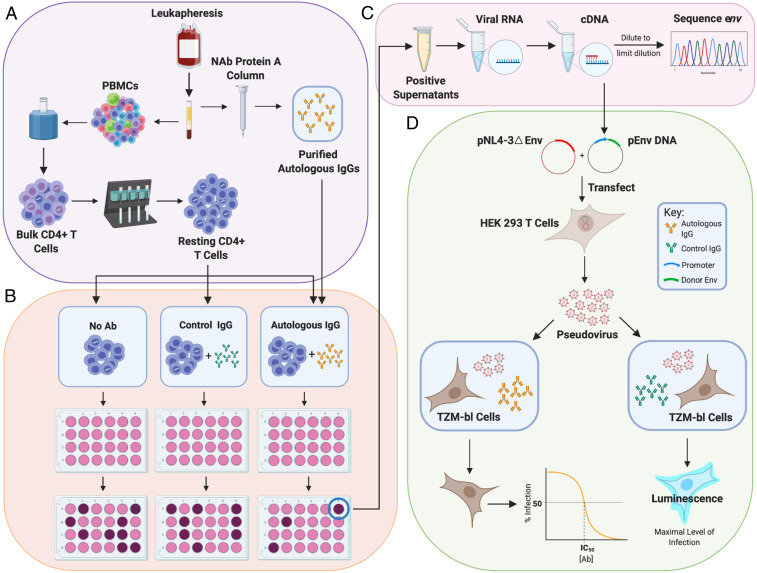
Fig. 1.
Evaluation of suppression of viral outgrowth by autologous neutralizing antibodies. (A) HIV-1–infected volunteers on long-term ART underwent leukapheresis, and resting CD4+ T cells and autologous IgG were purified from the same leukapheresis sample as described in Methods. (B) After an initial assessment of the frequency in intact proviruses by IPDA (65), resting CD4+ T cells were plated in limiting dilutions with no antibody, with control IgG from an HIV-negative donor (50 μg/mL), or with purified autologous IgG (50 μg/mL). Cells were then activated with phytohemagglutinin (PHA) and irradiated allogeneic PBMC according to the standard QVOA protocol (64). MOLT4/CCR5 cells were added to expand virus released following latency reversal. Enzyme-linked immunosorbent assays (ELISAs) for HIV-1 p24 on days 14 and 21 detected wells with viral outgrowth. (C) Viral RNA was isolated from p24+ supernatants, converted to cDNA, and diluted for SGS of the env gene. Sequences from isolates obtained in the presence of autologous antibodies were compared with sequences from isolates in control wells using phylogenetic analysis and neutralization assays. (D) For neutralization assays, cDNA was used to generate env-expression vectors for selected isolates. These were cotransfected into HEK 293 cells along with an env-deficient proviral construct to generate pseudoviruses. Pseudoviruses were incubated with autologous or control antibodies and then used to infect the TZM-bl reporter cell line (70, 71). Luminescence was measured at 72 h. Dose–response curves were analyzed as described in Methods.