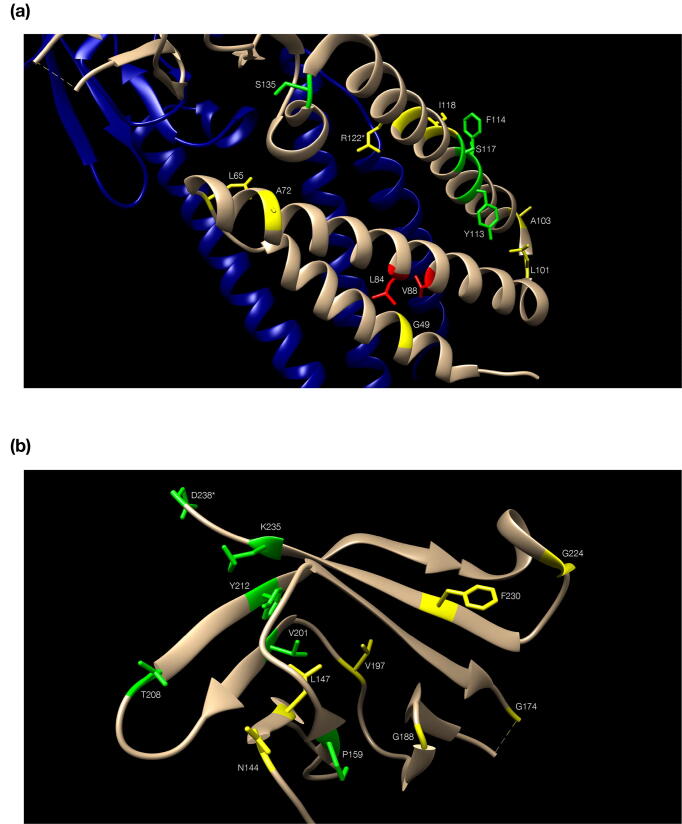
Fig. 3.
The role of conserved residues for the superfamily. Residues are color-coded in green (conserved) and yellow (quasi-conserved) (Table 1, [57]) with side chains shown only for those. Numbering follows the reference alignment. (a): the alpha-helical ectodomain of Orf3a, with the second subunit in blue to depict dimer configuration (orientation differs from Fig. 2b, to optimize labeling of residues); two non-conserved residues discussed in connection with the presence of G49 are colored in red. (b): the beta-sheet endodomain of Orf3a, starting at bottom (with N144) and ending at top (with D238); the outer (left) and inner (right) sheets are seen: strand β7 (starting at T208) participates in both; the α-helical turn following β7 is not marked as a helix (Fig. 2b). In both panels, two residues (R122 and D238) are marked by an *asterisk to signify detected steric clash issues. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)