Significance
During normal development, animals and their constituent body parts stop growing when they reach the right size. However, the mechanisms that limit growth as a function of size are largely unknown. Growth of the Drosophila wing, a classic paradigm, is governed by two morphogens, Decapentaplegic (Dpp, a BMP) and Wingless (Wg, a Wnt). We identify two constraints that cause the wing to cease growing when it achieves target size at the end of larval life: 1) an intrinsic limit on the spread of morphogen and 2) the capacity of the steroid hormone ecdysone to gate the ability of wing cells to grow in response to morphogen.
Keywords: Dpp Wg morphogens, organ size control, Drosophila wing growth, ecdysone gating, Hippo/Warts tumor suppressor pathway
Abstract
The stereotyped dimensions of animal bodies and their component parts result from tight constraints on growth. Yet, the mechanisms that stop growth when organs reach the right size are unknown. Growth of the Drosophila wing—a classic paradigm—is governed by two morphogens, Decapentaplegic (Dpp, a BMP) and Wingless (Wg, a Wnt). Wing growth during larval life ceases when the primordium attains full size, concomitant with the larval-to-pupal molt orchestrated by the steroid hormone ecdysone. Here, we block the molt by genetically dampening ecdysone production, creating an experimental paradigm in which the wing stops growing at the correct size while the larva continues to feed and gain body mass. Under these conditions, we show that wing growth is limited by the ranges of Dpp and Wg, and by ecdysone, which regulates the cellular response to their signaling activities. Further, we present evidence that growth terminates because of the loss of two distinct modes of morphogen action: 1) maintenance of growth within the wing proper and 2) induced growth of surrounding “pre-wing” cells and their recruitment into the wing. Our results provide a precedent for the control of organ size by morphogen range and the hormonal gating of morphogen action.
How do animals and their constituent body parts “know” to stop growing when they reach the right size? That they do is a remarkable feature of metazoan development, responsible for the highly stereotyped dimension and form of most animals. Yet, how this happens remains one of developmental biology’s enduring mysteries. Prevailing dogma invokes the existence of tissue-intrinsic stopping mechanisms that are engaged as organs approach their target size (1–4), a view that stems from classical transplantation and regeneration experiments. Famously, salamander limbs and eyes attain the correct size when grafted onto smaller or larger host species (5), as do mammalian kidneys (6), hearts (7), and skeletal elements (8) when cultured in novel environments. Likewise, diverse body parts of many animals regrow after tissue loss but typically cease growing when restored to normal size (9–12). Here, we ask why organs stop growing as a function of size, focusing on the Drosophila wing.
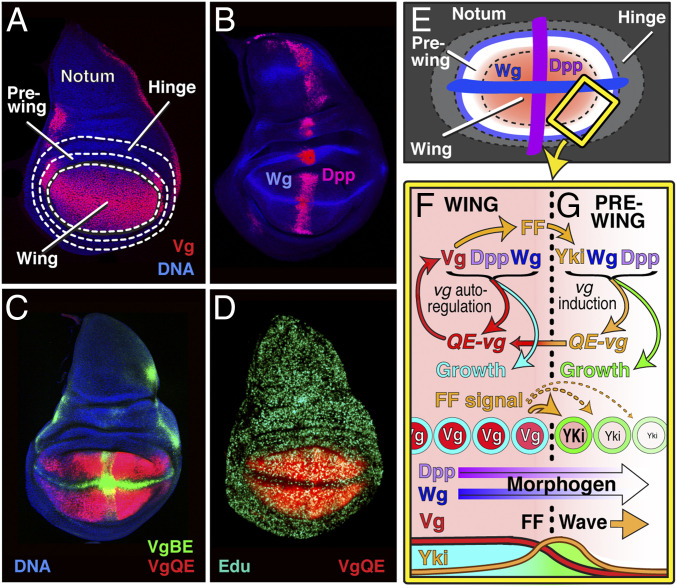
The wing develops during larval life as a distinct subpopulation of cells that express the selector gene vestigial (vg) within the wing imaginal disc (Fig. 1). The major phase of wing growth occurs during the second and third larval instars, a period of ∼3 d, when the primordium undergoes a dramatic ∼100-fold increase in cell number coupled to a corresponding increase in tissue mass. This growth is governed by two morphogens, Decapentaplegic (Dpp, a BMP) and Wingless (Wg, a Wnt), which are produced by orthogonal stripes of cells that are located, respectively, along the anteroposterior (A/P) and dorsoventral (D/V) compartment boundaries and intersect at the center of the wing primordium, the future distal tip of the adult wing (Fig. 1 B and E) (13–23). Previous evidence indicates that these molecules drive the rapid increase in wing size by at least two distinct mechanisms: 1) by sustaining growth as well as vg expression within the wing proper (Fig. 1F, turquoise mode) (13–23) and 2) by inducing growth and vg expression in surrounding “pre-wing” cells defined as such by their capacity to initiate Vg expression and enter the wing in response to Dpp and Wg (Fig. 1G, green mode) (19–21). Both mechanisms depend on the capacity of vg to autoregulate its own expression via a cis-acting “quadrant” enhancer (QE; Fig. 1 C and D) (13, 19, 20). In the first case, the autoregulatory circuit is intracellular and direct, requiring Vg to act on the QE to maintain vg transcription in wing cells in response to Dpp and Wg. In the second, it is intercellular and indirect, requiring wing cells to send a membrane-tethered, vg-dependent “feed-forward” (FF) signal, the protocadherin Fat, that induces QE-dependent vg expression in neighboring pre-wing cells, provided that they also receive Dpp and Wg (21). The Fat signal is received in pre-wing cells by a second protocadherin, Dachsous (21), and transduced via its transcriptional effector Yorkie (Yki) (24, 25). vg induction by Dpp, Wg, and the FF signal is reiterative: Once pre-wing cells turn on vg and enter the wing they become new sources of FF signal. Accordingly, as Dpp and Wg spread outward from the A/P and D/V boundaries, they fuel a propagating wave front of pre-wing growth and recruitment while also sustaining the continuing growth of wing cells behind (Fig. 1 F and G) (19–21).
Fig. 1.
Control of wing growth by Dpp and Wg. (A–E) Morphology, morphogen sources, and proliferative growth of the wing primordium. (A) The wing derives from a central population of cells within the wing imaginal disc that express the wing selector gene vestigial (vg), surrounded by concentric populations of pre-wing, hinge, and notum (body wall) cells as indicated (Vg protein, red; DNA, here and in the remaining figures counterstained with Hoeschst, blue). (B–E) vg expression is controlled by a cis-acting QE, which is activated in wing cells by Dpp and Wg emanating from source cells along the A/P and D/V compartment boundaries (magenta and blue, respectively, in B and E; QE activity is monitored by expression of a 5XQE.DsRed reporter gene, VgQE, red in C and D). vg is also controlled by a “boundary” enhancer (BE) activated by Notch signaling along the entire D/V boundary and in prevein territories in the mature wing, particularly in the vicinity of the A/P boundary (as monitored by a BE.lacZ reporter gene, vgBE, green in C). Notch signaling represses the QE, as visualized by the absence of 5XQE.DsRed expression along the D/V boundary and its partial suppression along the A/P boundary in D; hence, Vg expression in the entire wing disc (A) reflects the complimentary contributions of BE- and QE-dependent vg transcription. Growth of the wing proper is monitored by the near-uniform proliferative expansion of the population of QE-dependent Vg-expressing cells (monitored by EdU incorporation during S phase in D, turquoise). (F and G) Two distinct modes of growth that terminate when the wing reaches full size are diagrammed in the expanded region of the wing edge boxed in yellow in E (see ref. 21). In the first mode (G, right side), pre-wing cells encircling the wing are induced to grow (green), initiate QE-dependent Vg expression (orange), and enter the wing by the combined inputs of Dpp and Wg emanating from A/P and D/V border cells, as well as a membrane-bound, Vg-dependent FF signal, the protocadherin Fat. Fat operates via the Warts/Hippo tumor suppressor pathway to facilitate nuclear access of the transcriptional activator Yki, which acts directly on the QE enhancer. In the second mode (F, left side), Vg substitutes for Yki to autoregulate its own expression (red) and also programs wing cells to grow, both in response to Dpp and Wg (turquoise). Thus, as depicted at the bottom, the progressive outward spreads of Dpp and Wg (Morphogen) propel a wave front (arrow) of Yki activity, pre-wing growth, and recruitment that propagates away from the wing center, with the newly recruited wing cells serving as new sources of FF signal. Behind the front, Dpp and Wg retain cells within the wing proper (by fueling the autoregulation of Vg) and sustain their continuing growth, further expanding wing size.
Both pre-wing and wing growth terminate when the larval wing primordium reaches full size, but how remains unanswered despite intense investigation (1–4, 26). Notably, growth arrest is concomitant with the larval-to-pupal molt orchestrated by a surge followed by an abrupt decline in systemic 20-hydroxyecdysone (20E) (27, 28), the active form of the steroid hormone ecdysone (Fig. 2A). The roles of both the surge and decline in terminating growth are uncertain, as wings arrest normally, at full size, when transplanted into the abdomens of adult flies (1, 29), a context in which neither the surge nor abrupt decline in 20E occur (30, 31).
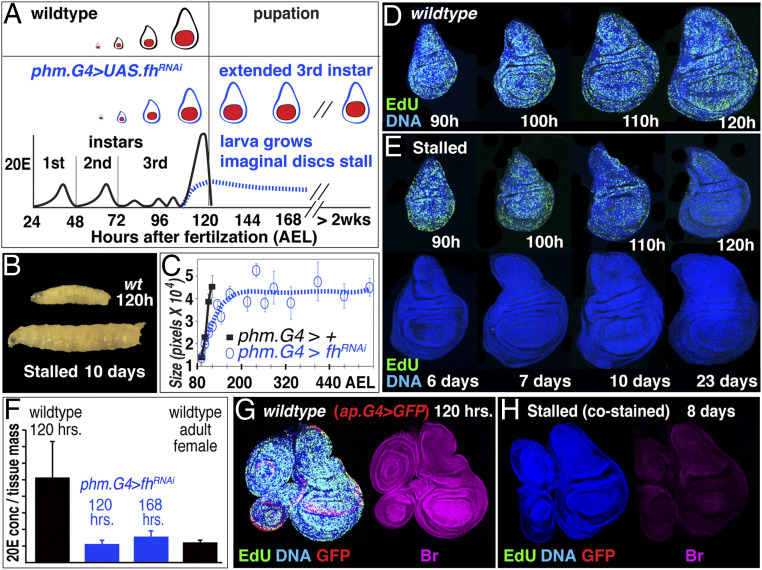
Fig. 2.
Generating “stalled” wing discs by compromising ecdysone (20E) production. (A and B) Stalled disc experimental paradigm. During normal development, 20E levels normally rise dramatically toward the end of the third larval instar, before declining abruptly at the onset of pupation, concomitant with the imaginal discs reaching full size (black, wing primordium shown in red) (28). Targeted knockdown of Fh in the larval prothoracic gland generated using either phm.Gal4+UAS.fhRNAi or phm.LexA+LexOP.fhRNAi causes an approximately three- to fourfold reduction in the late surge of 20E levels (A, dotted line, blue; quantified in F). Mutant larvae fail to pupate and continue to feed and grow (B). (C) Growth trajectories of stalled discs (phm.Gal4 UAS.fhRNAi; blue, open circles, dashed line) versus control discs (phm.Gal4; black, closed squares, solid line). In wing discs destined to stall, growth slows down during the second half of the third larval instar and ceases ∼1 to 2 d later than normal, after which the disc remains quiescent for extended periods of > 2 to 3 wk, until larvae cease feeding and die (disc size quantified by pixel number; Experimental Methods; n = 10 discs for each time point; error bars indicate SEM). (D and E) Proliferative growth in wild-type (D) versus stalled discs (E), as monitored by both the number of cells that incorporate EdU (and hence are in S phase) as well as the intensity of the signal (which reflects the rate of synthesis and declines as proliferative growth slows during the stall; SI Appendix; EdU, green; DNA, blue). (F) ELISA measurement of 20E concentration in wild-type larvae (late third instar, 120 h AEL), compared to phm.Gal4/UAS.fhRNAi larvae in which the discs have begun to stall or have completed the stall (120 and 168 h AEL), as well as with adult female abdomens. Error bars are SEM; n = 3 biological replicates for each treatment, each replicate contained 10 animals. (G and H) Expression of the protein product of the 20E target gene Broad (Br) provides an independent corroboration of reduced 20E transduction in stalled discs: Wild-type third instar imaginal discs express high levels of Br (G, magenta), in contrast to stalled discs from phm.LexA+LexOP.fhRNAi larvae, which show only low-level expression (H). Wild-type and stalled discs were treated identically but distinguished by ap.Gal4 driven expression of green fluorescent protein (GFP) in the wild-type discs (red; DNA, blue; EdU incorporation, green; SI Appendix).
Many organ-intrinsic mechanisms have been proposed to explain why the larval wing primordium (henceforth, the wing) stops growing when it reaches full size, including a decline in the grade (2, 32–34) or rate (35) of accumulation of Dpp and/or Wg, constraints on the ranges of these morphogens (19–21), the attainment of a complete set of intercalary positional cues (36, 37), or a buildup of negative molecular and/or mechanical feedbacks (3, 4, 26, 38, 39). Experimental support for these various mechanisms is scarce because the wing normally reaches full size at the onset of pupation, at the same time that it begins to metamorphose into the adult appendage, making it difficult to analyze why the newly quiescent cells have ceased proliferative growth. As a consequence, prior studies have investigated growth arrest in wing discs cultured ex vivo or as transplants in the abdomens of adult females, conditions in which metamorphosis does not occur. However, both contexts impose technical challenges that constrain analysis (29, 40–42). Here, we circumvent these challenges by using genetic means to curtail 20E production during normal development, creating larvae that cannot undergo the larval-to-pupal molt (43–45). Under these conditions, the imaginal discs grow to the right size but then become quiescent for weeks as the larvae continue to feed and grow. We show that the wing stops growing in such “stalled” discs because of limitations in the ranges of Dpp and Wg as well as the gating of the proliferative response of cells to Dpp and Wg by 20E.
Results
A Genetic Paradigm for Analyzing Growth Arrest.
To generate larvae that fail to pupate but instead continue to feed and gain mass after their imaginal discs cease proliferative growth, we used either Gal4/UAS (46) or LexA:VP16/LexOP (47) technology to drive RNA interference (RNAi) against the Drosophila homolog of Frataxin (Fh) in the larval prothoracic gland (43, 45)—the primary source of systemic 20E (both technologies are similarly effective and were used interchangeably depending on other genetic constraints; SI Appendix). Targeted RNAi knockdown of fh in the prothoracic gland reduces 20E production in the late third instar by approximately three- to fourfold (Fig. 2 A and F–H) and results in larvae that do not pupate but instead continue to feed and increase in body size for a vastly extended period of 3 to 4 wk (Fig. 2B). In contrast, growth of their imaginal discs, while initially normal, begins a progressive slowdown that starts ∼4 d after egg laying (AEL; approximately midway through the third instar in wild-type larvae) and ends when the discs reach full size at ∼6 to 7 d AEL (∼1 to 2 d later than normal; Fig. 2 C–E). At this point, they cease growing and enter a state of indefinite quiescence. Such “stalled” discs provide an experimental paradigm to identify the mechanisms that normally stop wing growth at the correct size, as manipulations that override these constraints should suffice to reinitiate growth. In this context, we show that two such constraints are morphogen range (which delimits pre-wing growth and recruitment; green mode in Fig. 1G) and gating of the capacity of wing cells to respond to morphogen by 20E (which dictates the growth of wing cells behind the FF recruitment front; turquoise mode in Fig. 1F).
Morphogen Range Limits FF Growth and Recruitment of Pre-wing Cells.
Given that the outward spread of Dpp and Wg promote wing growth at least in part by fueling the FF proliferation and recruitment of surrounding “pre-wing” cells (green mode, Fig. 1G) (19–21), we asked if growth at the wing periphery ceases in stalled discs because both morphogens have reached their maximum range. To do so, we increased their effective ranges by using a combination of Flp-out (48) and Gal4/UAS (46) techniques to pepper stalled discs with ectopic Dpp- or Wg-expressing cells (Fig. 3 and SI Appendix, Figs. S1 and S3). We focus specifically on the vg-expressing cells of the wing proper as well as the surrounding pre-wing cells that do not express vg but can be induced to do so by the combined inputs of Dpp, Wg, and the vg-dependent FF signal (19–21). To assay for an increase in the amount of wing tissue, we monitored the size and shape of the wing primordium as visualized by the expression of a 5X-vg-Quadrant Enhancer.DsRed (5XQE.Red) reporter or native Vg (Fig. 1 A, C, and D and SI Appendix, Figs. S1, S2, and S4) (19). To assay cell proliferation, we used the incorporation of 5-ethynyl-2′-deoxyuridine (EdU) during DNA synthesis to mark S phase nuclei, as well as the presence and size of “twin-spot” cell lineage clones (49) that were induced concomitantly with Dpp- or Wg-expressing clones (SI Appendix). Such twin-spot clones provide a “historical” record of proliferative growth following clone induction.
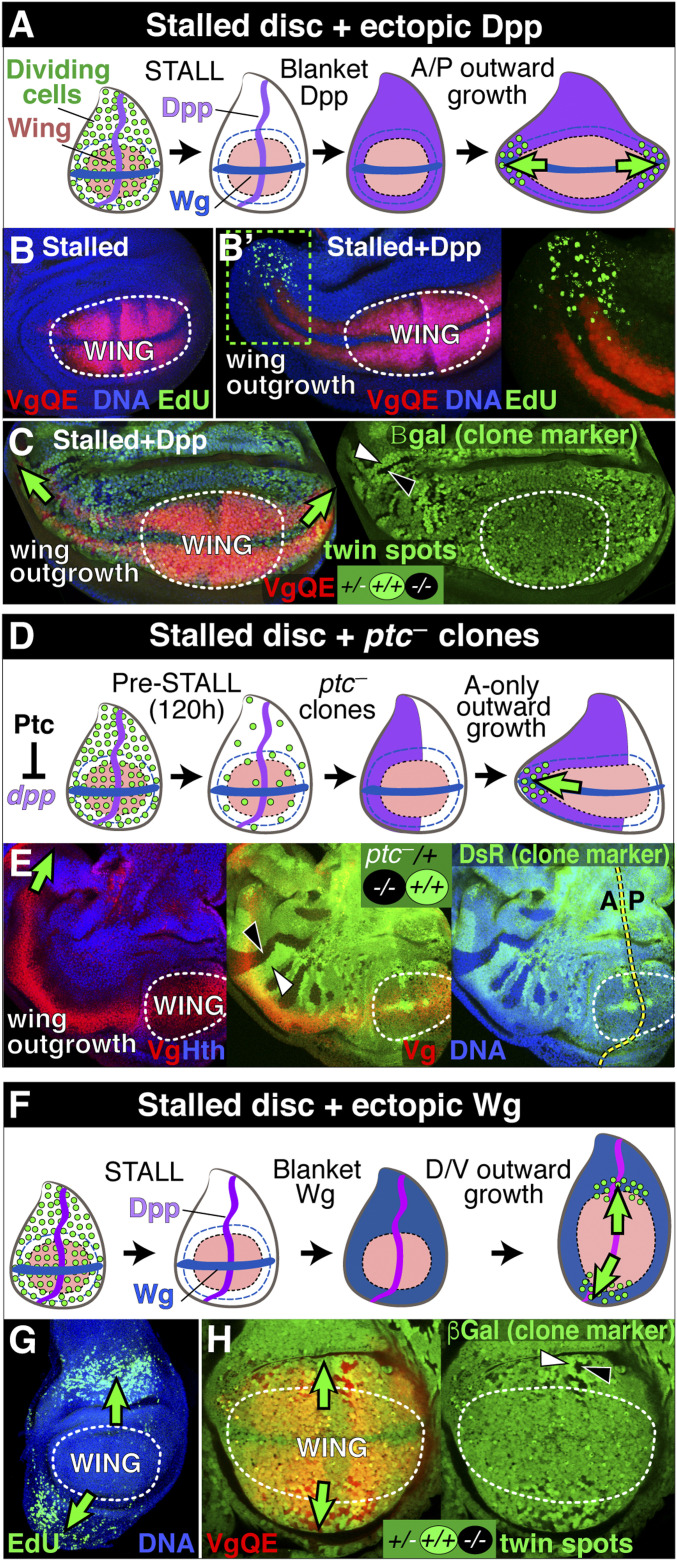
Fig. 3.
Reinitiation of wing growth by ectopic morphogen. (A–C) Blanket Dpp expressed under Gal4/UAS control. (A) Wing discs of larva carrying the phm.lexA:VP16, LexOP-fhRNAi, hsp70.flp, UAS > CD2 y+>dpp, C765.Gal4, and 5XQE.DsRed transgenes arrest growth (STALL) around 6 to 7 d AEL: heat-shock-induced excision of the >CD2 y+> cassette in most cells results in blanket Dpp signaling (magenta, confirmed by pMad staining; SI Appendix, Fig. S1) and reinitiation of proliferative growth in pre-wing cells at the anterior and posterior edges of the wing (depicted as dividing cells, green). (B) A stalled but otherwise control disc. The wing proper is encircled by a dotted line and marked by 5XQE.DsRed expression (red); no proliferative growth is apparent, as indicated by the lack of detectable EdU incorporation. (B′) Anterior portion of a stalled wing disc 3 d after the initiation of blanket Dpp signaling, stained and labeled as in B: proliferative growth (EdU, green) is apparent in pre-wing cells and newly recruited cells at the anterior edge of the expanding wing (boxed region; shown at higher magnification on the right) as it extends away from the center of the wing. Here and in E, G, and H the initial wing primordium at the time of the stall is encircled by a dotted line. (C) Stalled wing disc 3 d after the initiation of blanket Dpp containing twin-spot lineage-trace clones induced concomitantly with C765.Gal4 UAS > dpp-expressing clones (twin spots are marked by 0× and 2× expression of an arm.lacZ marker gene, green; an example is indicated by white (2×) and black (0×) arrowheads on the right). Twin spots are rare and small within the central portion of the wing, which existed at the time of clone induction, but common and increasing in size as a function of distance in the surround (see the main text). (D and E) Ectopic endogenous Dpp produced by clones of ptc— cells. (D) ptc— clones were induced 120 h AEL (approximately one to two cell divisions before growth arrest) in phm.lexA:VP16 LexOP-fhRNAi larvae that carry the hsp70.flp transgene and are also FRT42D ptc—/FRT42D Tub.DsRed. ptc— clones autonomously express peak physiological levels of Dpp in the A compartment (magenta), but not in the P compartment, resulting in the selective reinitiation of proliferative growth of pre-wing cells at the anterior edge of the wing as it extends away from the wing center (depicted as in A). (E) Anterior portion of a stalled wing disc ∼5 d after induction of ptc— clones, showing a dramatic expansion of the A compartment of the wing (green arrow; wing cells are visualized by Vg, red, and the absence of Homothorax [Hth], blue; ptc— clones are marked “black” by loss of a tub.DsRed transgene [DsR, green; black arrowhead] and their wild-type twin clones by 2× brighter expression of DsR [white arrowhead]; the A/P boundary is indicated by a dotted yellow line). No clones are observed in the central portion of the wing, or in the P compartment, but are apparent in the expanding A compartment, increasing in size as a function of distance from the wing center. (F–H) Blanket Wg (dark blue) expressed under Gal4/UAS control (as in A–C), except using a UAS > CD2 y+>wgNrt transgene (which expresses a membrane-tethered form of Wg that signals over a restricted range; SI Appendix). Proliferative growth (EdU, green, in G) reinitiates in pre-wing cells and neighboring hinge territories at the dorsal and ventral wing edges and expands outward, dorsally and ventrally (arrows), as corroborated by the size and frequency of twin-spot clones (black and white arrowheads in H).
Blanket Dpp signaling generated in stalled discs by widespread induction of Dpp-expressing cells (Fig. 3A; confirmed by general up-regulation of pMAD, SI Appendix, Fig. S1) reignited growth, resulting in a dramatic expansion of the wing primordium (Fig. 3 A–C and SI Appendix, Fig. S1). Strikingly, however, proliferation reinitiated—and then continued—only at the anterior and posterior edges of the expanding wing, as EdU incorporation was observed only in front and just behind the interface between the wing proper (marked by 5XQE.DSRed-expressing cells) and neighboring pre-wing cells (which do not express readily detectable 5XQE.DSRed; Fig. 3 B and B′). In accord, we observe twin-spot clones on both sides of the interface, increasing in size as a function of distance from the center of the wing, reflecting both proliferation of pre-wing cells in front of the interface and the recruitment of their descendants into nonproliferating wing tissue behind (Fig. 3C). By contrast, only small and less frequent twin spots were observed in the central portion of the wing, indicating little if any response of cells that already expressed vg at the time of the stall (Fig. 3C). Thus, the production of ectopic Dpp in stalled discs allows pre-wing cells to reinitiate proliferative growth, turn on QE-dependent vg, and enter the wing. Notably, however, pre-wing cells appear to respond to ectopic Dpp in this way only if they are also in position to receive Wg produced by cells along the D/V boundary, as well as FF signal from neighboring cells in the wing proper. This results in anterior and posterior wave fronts of proliferating, pre-wing cells that propagate away from the A/P boundary, leaving behind expanding populations of newly recruited, but mitotically quiescent wing cells in their wakes (SI Appendix, Fig. S3).
To determine if physiological levels of endogenous Dpp, rather than Gal4-driven levels, are sufficient to reinitiate wing growth in stalled discs, we induced ectopic expression of native dpp by manipulating Hedgehog (Hh) signal transduction. Normally, dpp expression is locally induced and maintained in A compartment cells along the A/P boundary in response to Hh secreted by P compartment cells (48, 50–52). Hh acts on its receptor Patched (Ptc), which is expressed only in A cells, to alleviate transcriptional repression of dpp (53). Accordingly, genetic ablation of Ptc mimics the reception of Hh signal in A cells but has no effect in P cells, inducing A—but not P—cells to ectopically express peak physiological levels of endogenous Dpp (53). We induced ptc— clones just prior to the stall to pepper the A, but not the P, compartment with cells that constitutively express native dpp (Fig. 3D and SI Appendix). As observed for cells that ectopically express Dpp under Gal4 control, ptc— clones in stalled discs cause a striking outward expansion of wing tissue, but in this case only from the anterior edge of the wing, as monitored by twin-spot clones, which increase in size as a function of distance from the A/P boundary (Fig. 3E). Also, as observed for ectopic Gal4-driven Dpp (Fig. 3 A–C), no growth was observed in the central portion of the wing, which remained quiescent (Fig. 3E). Finally, we corroborated these results by performing the complementary experiment of generating clones that ectopically express peak physiological levels of Dpp in the P rather than the A compartment (54) (SI Appendix, Fig. S2). Such clones cause a dramatic increase in wing tissue, but only at the posterior rather than the anterior edge of the wing.
Wg has been proposed to promote wing growth in an equivalent manner to Dpp, but along the D/V rather than the A/P axis (14, 19, 20, 55, 56, 21). Although the requirement for direct, long-range action of secreted Wg has recently been challenged (57), Wg nevertheless exerts long-range effects on vg expression and cell proliferation in the wing proper as well as on the growth and FF recruitment of the surrounding pre-wing cells (19–21). To test if the growth and recruitment of pre-wing cells is limited by the range of Wg as well as Dpp, we peppered stalled discs with clones that generate ectopic Wg signal (Fig. 3F and SI Appendix). As with blanket Dpp signaling, such indiscriminate Wg signaling drove the outward expansion of the wing. However, it did so along only the dorsal and ventral edges of the wing proper, where the responding cells are in position to receive Dpp, and not along the anterior and posterior edges where Dpp would be limiting (Fig. 3 F and G). As in the case of Dpp signaling, EdU incorporation was observed predominantly in cells in front of the interface with wing cells (Fig. 3G). Similarly, twin spots were found on both sides of the interface, increasing in size as a function of distance from the D/V boundary with only a few, small twin spots observed in central portions of the wing (Fig. 3H). Thus, ectopic Wg, like ectopic Dpp, appears to generate wave fronts of proliferating, pre-wing cells that propagate outward, albeit away from the D/V rather than the A/P boundary, leaving behind newly recruited, quiescent wing cells (SI Appendix, Fig. S3).
Taken together, these results show that Dpp and Wg can—and would—perpetuate growth at the wing periphery of stalled discs, if they were available. That no such growth occurs unless ectopic signal is provided indicates that the wing stops growing in stalled discs, at least in part, because of limitations in the ranges of Dpp and Wg. That the new growth induced by ectopic Dpp or Wg is restricted predominantly to pre-wing cells located in front of the expanding wing primordium argues further that morphogen range limits wing growth by restricting where the FF recruitment mechanism can operate (SI Appendix, Fig. S3). We test this possibility next by assaying the consequences of ectopically activating the FF pathway in stalled discs.
Access to FF Signal Limits Pre-wing Growth.
The FF signal, Fat, acts by downregulating the NDR kinase Warts (Wts), the key effector kinase of the Hippo/Warts tumor suppressor pathway (58), in pre-wing cells, allowing the transcription factor Yki (24, 25) to enter the nucleus and initiate the positive autoregulation of vg in response to Dpp and Wg (21). Hence, the FF transduction pathway can be constitutively activated by reducing or eliminating Wts. Accordingly, if wing growth in stalled discs is limited by the availability or intensity of FF signal, blanket reduction of Wts activity should overcome growth arrest in pre-wing cells provided that they are also in position to receive Dpp and Wg.
To test this, we again used RNAi knockdown of fh in the prothoracic gland to generate stalled discs, only this time in larvae in which wts activity is reduced by mutation (Experimental Methods). As shown in Fig. 4 A and B, wing growth does not cease in such wts— stalled discs. Instead, the wing primordium continues to expand in dramatic fashion. Nevertheless, as observed for ectopic Dpp and Wg, proliferation occurs only at the wing periphery, with the surrounding cells incorporating EdU until they initiate vg expression and are recruited into the wing (Fig. 4 D and F). Markedly, the wing expands predominantly along the D/V axis, suggesting that growth along the A/P axis is constrained by the limited range of Dpp coming from the A/P boundary. Conversely, the lack of constraint along the D/V axis suggests that growth in this axis does not depend on Wg emanating from the D/V boundary but instead on Wg coming from elsewhere. A candidate source is the “inner ring” (IR) of wg-expressing cells located in the surrounding wing hinge primordium (Fig. 4C). These cells are normally located several cell diameters from the wing proper and separated from it by a deep fold. Hence, we posit that pre-wing cells that are located close enough to receive Wg from the IR in otherwise wild-type stalled discs are normally positioned too far from vg-expressing cells in the wing proper to receive FF signal, preventing them from being induced by Dpp and Wg to grow and enter the wing. However, when wts activity is reduced, these cells experience constitutive Yki activity (mimicking receipt of ectopic FF signal) in addition to high levels of Wg from IR cells, and hence should be limited only by the availability of Dpp (Fig. 4C). Indeed, it is specifically pre-wing cells that appear to include and surround the IR and are in position to receive Dpp that comprise the proliferating wave fronts in wts— stalled discs and give rise to the rapidly expanding population of newly recruited wing cells (Fig. 4 D and F).
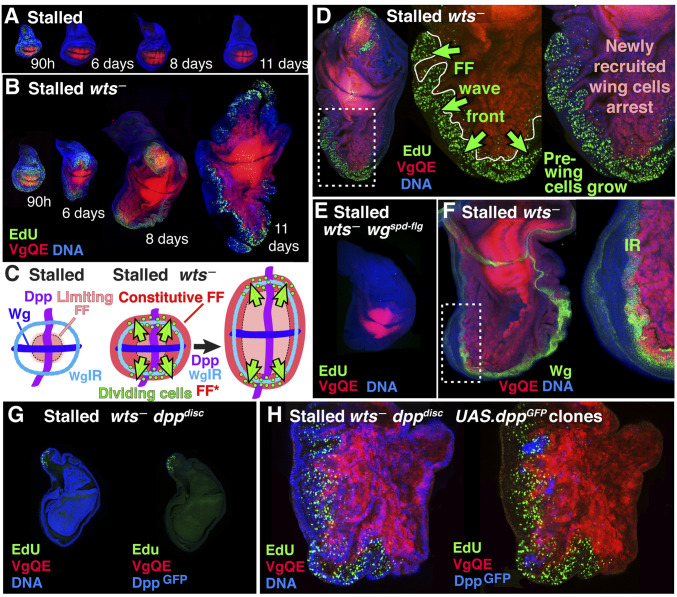
Fig. 4.
Constitutive Yki activation sustains morphogen-dependent growth and recruitment of pre-wing cells. (A and B) Constitutive Yki activity sustains FF growth in stalled discs. In contrast to wing discs of phm.lexA:VP16 LexOP.fhRNAi but otherwise wild-type larva, which stall after ∼6 to 7 d (A), wing discs from phm.lexA:VP16 LexOP.fhRNAi larvae that are homozygous for the wtsP2 mutation have constitutive Yki activity and show continuous proliferative growth of cells at the wing periphery (monitored by EdU incorporation, green, in both A and B), as well as a dramatic expansion in the wing proper, primarily in the D/V axis (5XQE.DsRed, red). As observed for blanket Dpp and Wg expression in stalled discs, growth is restricted to the wing periphery: Once cells initiate vg expression and enter the expanding wing primordium they arrest. (C–F) Continuous FF growth in stalled wtsP2 larvae depends on Wg produced by IR cells surrounding the wing primordium. (C) In wtsP2 stalled discs, wing growth by FF propagation should be limited only by the availability of Dpp and Wg but is observed far outside of the expected range of Wg produced by D/V border cells. However, IR cells (WgIR, light blue) provide a second, potential source of Wg. In stalled but otherwise wild-type discs, we envisage that pre-wing cells that are in position to receive this Wg as well as Dpp do not respond because they are located too far from the wing proper to receive FF signal. By contrast, in stalled wtsP2 discs, these pre-wing cells should now receive constitutive FF input in addition to Wg produced by IR cells and hence should continue to grow and be recruited into the wing, provided they also receive Dpp. As observed (D and F), it is specifically non-Vg-expressing cells in the immediate surround of the expanding wing primordium that are exposed to Wg from the IR (F, green, IR) and continue to proliferate (D). No wing growth is observed in wing discs from stalled, wtsP2 larvae that are also homozygous for wgspd-flg, an enhancer mutant allele of wg that selectively eliminates Wg expression in IR cells (E), confirming that these cells are the source of Wg responsible for the continuous growth at the wing periphery in wtsP2 stalled discs. (G and H) Continuous wing growth in stalled wtsP2 discs depends on Dpp. No growth is observed in stalled, wtsP2 wing discs that are transheterozygous for two dppdisk enhancer mutant alleles, dppd8 and dppd10, that abolish Dpp expression in the wing disc (G); however, continuous growth can be reinitiated in such discs by heat-shock-induced excision of the stop cassette of an Act > stop > Gal4 transgene to generate clones of UAS.dppGFP cells that ectopically express a GFP-tagged form of Dpp (H, blue; SI Appendix).
To test this interpretation, we generated wts— stalled discs that are also homozygous for wgspdfg, a wg enhancer mutant allele that selectively abolishes the IR of wg expression (59). In wgspdfg wts— stalled discs, we observe no EdU incorporation at the wing periphery and little or no expansion of the wing primordium (Fig. 4E; see also ref. 60) despite the fact that pre-wing cells along the dorsal and ventral edges of the primordium should be receiving both maximal Dpp and constitutive Yki input. These results corroborate the IR cells as the source of Wg responsible for fueling the perpetual outward expansion of the wing in wts— stalled discs.
To test if this outward expansion is similarly dependent on Dpp coming from A cells along the A/P compartment boundary, we used dppdisk mutant alleles (61) to abolish endogenous Dpp expression in wts— stalled discs and then assayed the consequences of inducing clones of ectopic Dpp-expressing cells. dppdisk wts— stalled discs appear to lack a wing primordium (Fig. 4G), and the remaining portions of the disc terminate growth around the time otherwise wild-type discs would stall, even though any pre-wing cells that remain should be exposed to high Wg signal and constitutive Yki activity. However, peppering such discs with ectopic Dpp-expressing cells after they stall suffices to initiate the recovery of an otherwise cryptic wing primordium that now expands outward from the wing periphery (Fig. 4H).
We conclude that the continuous expansion of the wing in wts— stalled discs, like that in ectopic Dpp- or Wg-expressing stalled discs, results predominantly if not exclusively from the growth and recruitment of pre-wing cells that receive the trifecta of Dpp, Wg, and Yki input.
Ecdysone Gates the Growth of Wing Cells behind the FF Recruitment Interface.
The above results indicate that the wing stops growing in stalled discs at least in part because of a failure to sustain the FF wave front. Specifically, as the wing increases in size, the outward spreads of Dpp and Wg from their respective sources along the A/P and D/V boundaries reach their maximum range and the surrounding pre-wing cells lose access to one or both signals, terminating their capacity to proliferate and enter the wing (green mode of growth in Fig. 1G). Strikingly, the growth of cells behind the FF recruitment interface (turquoise mode in Fig. 1F) also terminates in stalled discs—even though they no longer require the FF signal and are in position to receive both morphogens. This is particularly notable for pre-wing cells of stalled discs that grow and enter the wing in response to ectopic Dpp, Wg, or Yki activity: They become quiescent as soon as they initiate vg expression, despite continuing to receive both Dpp and Wg (as corroborated by their QE-dependent expression of Vg; e.g., Figs. 3 and 4). Accordingly, we posit that cells within the wing proper are programmed by Vg to require a critical threshold of 20E to grow in response to Dpp and Wg. Hence, we propose that just as growth in front of the FF recruitment interface is limited by morphogen access, growth behind is gated by systemic 20E. Wing cells grow in response to Dpp and Wg when the 20E gate is open. However, when 20E levels fall beneath a critical threshold, the gate closes and wing cells arrest, regardless of morphogen input.
A prediction of this hypothesis is that experimentally restoring 20E to stalled discs should reopen the gate and reignite growth of the wing proper. As an initial test, we fed stalled larvae with exogenous 20E. Strikingly, doing so induced a global resumption of proliferation in the entire disc, including within the wing (Fig. 5A). The same result was also obtained in wts— stalled discs: Resupply of 20E caused cells throughout the vastly expanded, but mitotically quiescent, population of wing cells to resume proliferative growth (SI Appendix, Fig. S4 C and D).
Fig. 5.
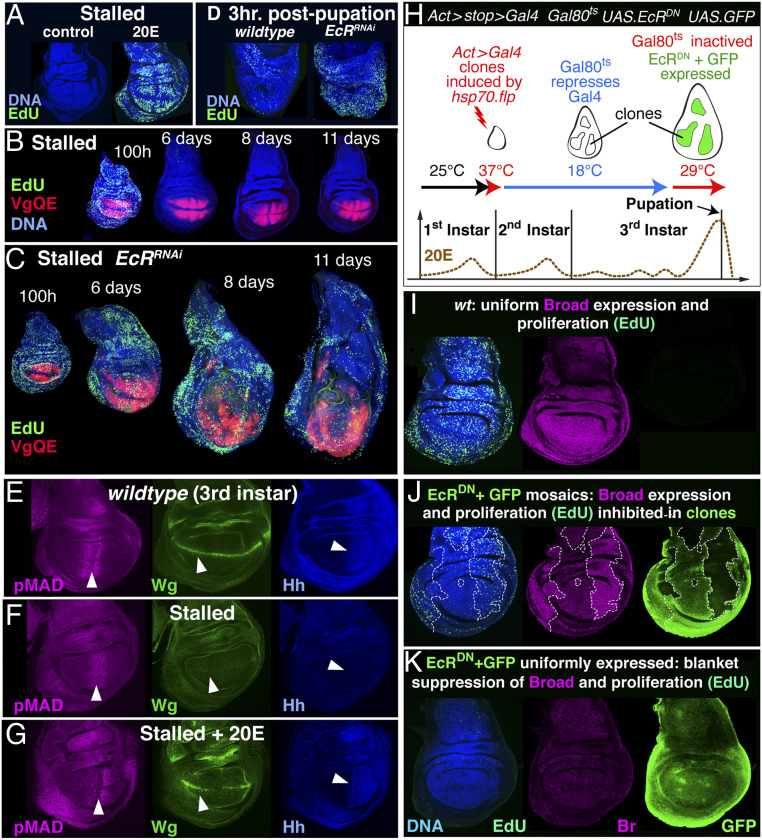
Gating of wing growth and morphogen production by 20E. (A) Feeding 20E to phm.lexA:VP16 LexOP.fhRNAi larvae for 18 h causes cells throughout stalled discs to reinitiate growth, as monitored by EdU incorporation (green). (B and C) Likewise, targeted activation of the 20E transduction pathway in wing discs of phm.lexA:VP16 LexOP.fhRNAi larvae sustains continuous growth including in the wing proper (marked by 5XQE.DsRed, red; activation was achieved using the C765.Gal4 transgene to drive imaginal disc-specific expression of a UAS.EcRRNAi transgene; the UAS.EcRRNAi expressing phm.lexA:VP16 LexOP.fhRNAi larvae continue to feed and gain mass like control phm.lexA:VP16 LexOP.fhRNAi larvae and the discs do not evert, indicating that they do not initiate metamorphosis). (D) Constitutive activation of the 20E transduction pathway within the wing primordia of otherwise wild-type larvae (by rn.Gal4 driven expression of UAS.EcRRNAi) sustains wing growth at least 3 h past the onset of pupation when it would otherwise cease. (E–G) Morphogen production is reduced in stalled discs owing to diminished 20E. Phosphorylated MAD (pMad, a readout of Dpp transduction, magenta), Wg (green), and Hh (blue) expression in wild-type mature third instar discs (E), 9-d-old stalled discs (F), and 9-d-old stalled discs from larvae fed 20E for 18 h (G). Arrowheads indicate positions of peak Dpp, Wg, and Hh production in E and the corresponding positions in F and G, as monitored, respectively, by the intensity and width of the stripe of pMad staining, Wg accumulation along the D/V boundary, and Hh accumulation throughout the P compartment [the local trough in pMad staining along the A/P boundary in E and G is due to peak Hh signaling (87)]. All three signals are significantly reduced in stalled discs (F; see also SI Appendix, Fig. S3) but partially or fully restored in stalled discs after 20E feeding (G). (H–K) Suppressing 20E transduction during normal wing development causes premature growth arrest. (H) Experimental scheme for blocking the 20E signal transduction pathway in large clones of cells, or all cells, in otherwise wild-type wing discs using Flp-out and Gal4/Gal80ts technology to control expression of a dominant negative form of the EcR (EcRDN). Larvae carrying the transgenes hsp70.flp, Act > C2D > Gal4, Tub.Gal80ts, UAS.EcRDN, and UAS.GFP were heat-shocked at 48 h AEL to generate isolated cells carrying the “flipped-out” Act > Gal4 transgene following excision of the >C2D> stop cassette. Larvae were then transferred to 18 °C, permitting Gal80ts to inhibit Gal4-driven expression of EcRDN in the clonal descendants of these cells. Mid-third instar larvae (corresponding to wild-type larvae at ∼96 h AEL at 25 °C) were then shifted to 29 °C to abolish Gal80ts activity and initiate EcRDN expression, and the discs fixed and dissected 24 h later. (I) Proliferative growth (Edu, green) and activity of the 20E transduction pathway (monitored by Br expression, purple) in a mature, wild-type wing disc; Br is strongly expressed, particularly in the wing and hinge primordia. (J) A mature disc containing large clones of cells expressing EcRDN (marked by GFP, green): Br expression is diminished and proliferative growth arrested within the clones. (K) A disc in which all cells carry the flipped-out Act > Gal4 transgene and express UAS.EcRDN; Br expression is reduced and cell proliferation blocked throughout.
To test whether 20E acts directly on wing cells to control their capacity to grow, we cell-autonomously activated or blocked the 20E signal transduction pathway in these cells. Transduction of 20E depends on a heterodimeric nuclear receptor complex composed of the Ecdysone Receptor (EcR) and Ultraspiracle (Usp) that normally act to repress a subset of 20E target genes in the absence of hormone (62). Consequently, these genes are derepressed by targeted RNAi knockdown of either EcR or Usp (63), resulting in a constitutive, albeit partial, activation of the pathway. Conversely, 20E transduction can be blocked by the expression of a dominant negative form of EcR that cannot respond to ligand (EcRDN) (64). Targeted RNAi knockdown of either EcR or Usp in stalled imaginal discs (using the imaginal disc-specific driver C765.Gal4) mimics the consequence of providing exogenous 20E by feeding, but in a manner that is autonomous to the discs (the larvae do not pupate, but instead continue to feed and gain mass, as in the standard stalled disc paradigm). Disc growth is negligibly affected up until the time the stall would normally begin (∼90 h AEL); however, such discs fail to stall and cells throughout, including in the prospective wing, continue to proliferate (Fig. 5 B and C and SI Appendix, Fig. S4 A and B). Hence, it appears that cells in the wing proper stop growing in stalled discs because the 20E level falls beneath a critical threshold.
During normal development, 20E levels rise to peak levels toward the end of larval life and wing growth terminates abruptly, coincident with a precipitous decline in 20E and the onset of pupation. To assess if this decline plays a causal role in the arrest of wing cells under normal conditions, as our evidence indicates it does in the stalled disc paradigm, we performed two experiments. First, we used targeted RNAi knockdown of EcR to constitutively activate the 20E transduction pathway in the prospective wing of otherwise wild-type larvae (using the rn.Gal4 driver, which acts selectively in both wing and pre-wing cells). Doing so caused wing cells to continue proliferating past the time they would otherwise have arrested (Fig. 5D). Second, we performed the reciprocal experiment of using EcRDN expression to inhibit 20E transduction during the latter half of the third larval instar, when 20E levels normally rise to peak level. To do so, we used Gal80ts/Gal4 technology (65) combined with an Act5C > stop > Gal4 transgene to conditionally turn on EcRDN expression in large, early-induced Act5C > Gal4 “Flp-out” clones or in entirely Act5C > Gal4 wing discs (Fig. 5H). In both cases, wing cell proliferation (as monitored by EdU incorporation) terminated autonomously within 24 h of the onset of EcRDN expression (Fig. 5 I–K).
We conclude that the capacity of wing cells to proliferate, both in stalled discs as well as under normal conditions, is gated by 20E: Wing cells must receive a critical level to grow in response to Dpp and Wg; growth ceases when 20E transduction falls beneath this threshold. Significantly, blocking 20E transduction before pupation causes precocious arrest of pre-wing as well as wing cells (Fig. 5 J and K), indicating that pre-wing cells also require a minimum threshold of 20E to grow. However, we infer this threshold is lower than that required by cells within the wing proper, as the level of systemic 20E that persists in the stalled disc paradigm allows pre-wing cell proliferation to be reignited and sustained by ectopic Dpp, Wg, or Yki even as cells that are recruited into the wing are consigned to a state of indefinite quiescence.
Ecdysone Gates Morphogen Production.
Based on the preceding results, we envisage that wing growth is terminated in stalled discs by at least two mechanisms: 1) limitations in the ranges of both Dpp and Wg that prevent FF growth and recruitment of pre-wing cells and 2) the closing of the 20E gate on the capacity of wing cells to grow in response to morphogen. That both modes of growth cease simultaneously at the onset of pupation further suggests that they might be coupled, a possibility that could be achieved if morphogen production were itself gated by 20E.
To test this, we compared Dpp and Wg signaling in wild-type versus stalled discs. In wild-type discs, Dpp and Wg, as well as their upstream activators, signal at peak levels during the third larval instar, the major period of wing growth. In contrast, we observe a progressive reduction in expression and/or signaling by all of these factors in discs destined to stall, beginning around ∼24 to 36 h before the stall and extending for several days thereafter. In the case of Dpp, this is apparent in the narrowing of the stripe of cells that accumulate pMad, a meter of Dpp transduction, as well as in reduced signaling by its upstream inducer, Hh, as monitored by reductions in both Hh abundance (Fig. 5 E and F) and Ptc expression [an indicator of Hh signaling intensity (53); SI Appendix, Fig. S5 A and C]. Likewise, Wg expression as well as that of one of its inducers, the Notch ligand Delta (66), also decline in stalled discs (Fig. 5 E and F and SI Appendix, Fig. S5 A and C). Conversely, feeding larvae 20E after the stall reversed these declines, restoring Dpp, Hh, and Wg to levels approaching those found in normal third instar discs (Fig. 5H). Importantly, manipulating 20E levels in stalled discs did not generally compromise protein biosynthesis, as the levels of other proteins such as Yki remain unaffected, while, conversely, levels of the EcR-B1 isoform increased (SI Appendix, Fig. S5A).
Hence, in addition to gating the capacity of wing cells to grow in response to morphogen, 20E also appears to gate their capacity to produce morphogen, providing a possible means to limit morphogen range and thereby couple termination of the FF growth and recruitment of the pre-wing with growth arrest of the wing proper.
Discussion
Despite intense study, the yin-and-yang relationship between tissue growth and its termination remains poorly understood. In some systems, such as the Drosophila wing, we know that BMPs and Wnts control growth. Yet, how they do so and how their actions are limited so that organs stop growing at the correct size are unknown. Here, we have sought to identify constraints on BMP (Dpp) and Wnt (Wg) action that terminate growth by asking what factors can reignite growth after it has ceased. Our results indicate two such constraints: 1) the maximum ranges of Dpp and Wg from their normal sources within the wing and 2) hormonal gating of the capacity of cells to respond to, and possibly to produce, these morphogens.
Morphogen Range Limits Organ Size.
To identify factors that can reignite growth after the wing reaches full size, we compromised production of 20E, the active form of the steroid hormone ecdysone; doing so blocks the pupal molt and results in “stalled” wing imaginal discs that stop growing at the correct size while the larva continues to feed and gain mass. Under these conditions, we show that growth of the prospective wing can be reinitiated by ectopically expressing either Dpp or Wg, albeit only at the wing periphery, and only in regions far from the normal source of the ectopically expressed morphogen. Further, we provide evidence that this new growth occurs by a previously proposed mechanism in which wing cells send a short-range FF signal that induces neighboring pre-wing cells to proliferate and enter the wing in response to Dpp and Wg (Figs. 1G, 3, and 4) (19–21). This process is reiterative: Once pre-wing cells enter the wing they become new sources of FF signal, generating a wave front of proliferative growth that propagates outward fueled by the spread of Dpp and Wg (SI Appendix, Fig. S3). Importantly, by showing that the FF mechanism is normally limited by the maximum range of morphogen action, our results link the arrest of this mode of growth to tissue dimension.
Control of Organ Size by Hormonal Gating of Morphogen Action.
During normal larval life, the outward spread of Dpp and Wg controls wing growth not only by propelling the growth and recruitment of pre-wing cells ahead of the FF wave front (green mode, Fig. 1G) but also by sustaining the growth of wing cells behind (turquoise mode Fig. 1F). This second mode is not normally limited by morphogen range, as all cells within the wing proper receive sufficient Dpp and Wg to maintain expression of vg, which selects the wing state (Fig. 1F). Instead, we provide evidence that it is limited by 20E titer. Wing cells cease growing in discs that are destined to stall owing to inadequate 20E, but reinitiate growth in response to the resupply of 20E or constitutive activation of the 20E transduction pathway (Fig. 5 A–D and SI Appendix, Fig. S4). Reciprocally, blockade of the 20E transduction pathway causes premature growth arrest (Fig. 5 H–K). Hence, we conclude that under normal conditions 20E may gate wing growth by sustaining the capacity of wing cells to proliferate and gain mass in response to morphogen, consistent with 20E serving both growth-promoting as well as growth-terminating roles, depending on developmental context (67–71).
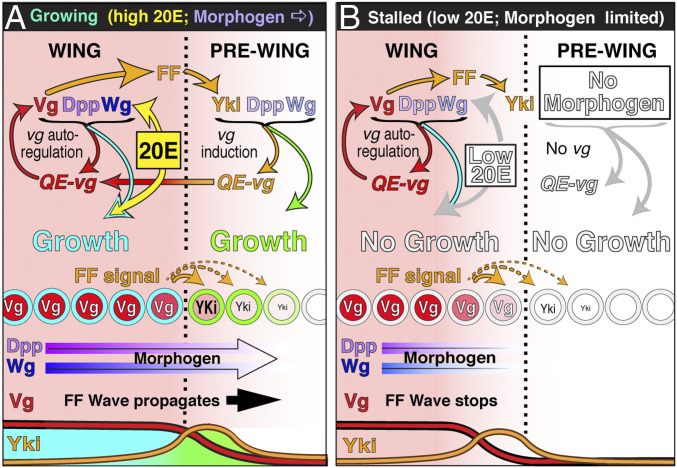
Notably, 20E levels decline slowly over a prolonged period in the stalled disc paradigm, rather than precipitously as they do at the end of normal larval life, creating a situation in which growth appears to arrest in “slow motion” as Dpp and Wg reach their maximal ranges (limiting any further growth and recruitment of pre-wing cells in front of the FF interface) and 20E levels fall beneath a critical threshold (limiting any further growth of cells in the wing proper, behind the interface). Under these conditions, we observe that Dpp and Wg production wanes as discs stall but rebounds to normal levels when growth resumes in response to exogenous 20E (Fig. 5 E–G and SI Appendix, Fig. S3). Hence, morphogen production appears to depend on 20E titer. Accordingly, the decline in 20E levels at the end of larval life, whether abruptly under normal conditions or slowly in the stalled disc paradigm, should limit morphogen range, providing a potential mechanism to couple the growth arrest of pre-wing cells in front of the FF recruitment interface with that of wing cells behind (Fig. 6).
Fig. 6.
Control of wing size by morphogen range and hormonal gating. (A) In wild-type third instar (control) discs, Dpp and Wg fuel a propagating wave front of pre-wing growth (green) and FF-induction of Vg (orange) that recruits new cells into the growing wing while at the same time sustaining Vg expression (red) and the proliferative growth of wing cells behind (turquoise) (Figs. 1 G and H; as in ref. 21). We posit that pre-wing growth and recruitment is normally limited by the capacity of Dpp and Wg to spread, whereas growth of the wing proper is limited by the requirement for sufficient 20E (yellow) to allow wing cells to grow in response to Dpp and Wg; 20E also appears to gate the production of both morphogens. Accordingly, 20E may promote pre-wing growth and recruitment by increasing morphogen production at the same time that it sustains the capacity of wings to grow in response. Conversely, the abrupt decline of 20E at the end of larval life might couple arrest of pre-wing growth with that the wing proper by simultaneously curtailing the production of Dpp and Wg as well as the capacity of wing cells to respond. (B) In wing discs destined to stall, 20E levels are dampened, and the subsequent decline is greatly prolonged, imposing the same constraints on pre-wing and wing growth, but in a gradual rather than abrupt manner. As a consequence, FF growth and recruitment of the pre-wing slows down and arrests at the limits of Dpp and Wg range (No Morphogen), and growth of the wing proper ceases concomitantly as 20E levels fall below the threshold necessary for wing cells to proliferate (Low 20E).
Organ Intrinsic versus Systemic Mechanisms for Growth Arrest.
Our hormonal gating model contrasts with the prevailing dogma that growth arrest is controlled predominantly by organ-intrinsic stopping mechanisms (1–4, 26). Prior evidence for this view in Drosophila comes from classical transplantation experiments in which imaginal discs were cultured in the abdomens of adult females. Under these conditions, the discs grow to full size and arrest as in wild-type larvae, consistent with growth being terminated by disc autonomous mechanisms (1, 29). However, systemic 20E is present at low levels in adult females, as it is in larvae with stalled discs (30, 31) (Fig. 2F). Hence, both experimental contexts represent similar humoral settings in which the 20E gate may be closed enough to prevent cells within the wing proper from growing, but sufficiently open to allow pre-wing cells to grow and enter the wing if they have access to Dpp, Wg, and the FF signal. Indeed, transplanted discs can be induced to reinitiate wing growth by surgical rearrangements, a manipulation we infer exposes pre-wing cells to Dpp or Wg they would otherwise not receive (29, 72–74), thereby mimicking the expression of ectopic morphogen in stalled discs. Similarly, as in wts— stalled discs, the wing primordium continues to expand in transplanted discs in which the FF signal transduction pathway is constitutively activated (29, 40, 75). Thus, corresponding manipulations of morphogen and FF signaling yield similar evidence for organ-intrinsic limits to growth in both experimental contexts. What distinguishes our present findings is that we have also been able to assay the consequences of manipulating 20E signaling. Doing so has allowed us to identify hormonal gating as a significant, organ-extrinsic constraint on growth, operating through its regulation of organ-intrinsic morphogen action. We therefore suggest that our findings do not conflict with prior evidence for organ-intrinsic stopping mechanisms but instead provide a more general explanation.
Other Limits to Growth.
Although our findings indicate that morphogen range and hormonal gating limit wing growth, they are unlikely to be the only such constraints. Indeed, the remarkable precision with which the wing stops growing when it reaches the correct size can best be explained if these constraints work in concert with negative-feedback mechanisms that operate either via the control of morphogen action or by other means (26). For example, as established for Hh (53), both Dpp and Wg may restrict their own abilities to move through responsive tissue by modulating the expression of their receptors or other binding proteins (76–80), thus delimiting their maximum ranges. Wing growth also appears to be constrained by an endocrine feedback circuit in which developing discs secrete Drosophila insulin-like peptide 8 (Dlp8) to control 20E titer (67, 81–84, 70, 85) and hence regulate the production and activities of morphogens like Dpp and Wg upon which their growth depends.
However, our results challenge other mechanisms that have been proposed to limit wing growth, in particular those in which a decrease in the grade (2, 32–34) or rate (35) of morphogen accumulation, or alternatively, an increase in mechanical tension caused by the compaction of cells (38, 39), serve as indicators of organ dimension. For the first class of models, exposing stalled discs to ubiquitous morphogen would be expected to flatten any residual spatial and temporal differentials in signaling and hence reinforce rather than override growth arrest. Yet, subjecting stalled discs to blanket Dpp or Wg reinitiates and sustains a continuous expansion in wing size, belying these expectations but according well with models in which organ size is governed by morphogen range. Likewise, for the second class, it is unclear how blanket morphogen exposure would overcome growth arrest imposed by mechanical constraints, unless these constraints are alleviated by the expanded range and action of morphogen on the cells that impose them.
Might morphogen range and hormonal gating of morphogen action limit the growth of organs other than the wing? The identity and roles of the morphogens that control the development of other portions of the fly, such as the eyes, antennae, and legs, and even other derivatives of the wing imaginal disc (e.g., the notum; Fig. 1), are much less well understood than in the case of the wing. Nevertheless, we have observed that the imaginal disc primordia giving rise to these other body parts arrest at full size in the stalled disc paradigm and, like the wing, begin growing again when provided with ectopic morphogen, loss of Wts activity, or activation of the 20E signal transduction pathway. Although the logic responsible for reigniting growth in these other contexts remains to be elucidated, these findings nevertheless suggest that morphogen range and hormonal gating play a general role in the control of organ size in Drosophila. Perturbations of hormone physiology alter organ size and shape in diverse animals, including humans (86). We speculate that at least some of these alterations are due to abnormal gating of morphogen production or action, consistent with a fundamental role for such gating in the control of growth.
Experimental Methods
Generation of Stalled Discs.
To generate larvae with imaginal discs destined to stall, we compromised 20E production by using phm.Gal4 or phm.LexA:VP16 transgenes to drive UAS.fh or LOP.fh expression in the prothoracic gland (43, 45) (SI Appendix). To assess the consequences of ectopic morphogen, reduced Wts function, or gain or loss of 20E transduction in stalled discs, we introduced the required genetic elements (SI Appendix, Table S1) by standard genetic crosses allowing identification of larvae of the desired genotypes (SI Appendix, Table S2).
Analysis of Stalled Discs.
Antibody staining, EdU labeling of S-phase nuclei, quantification of disc growth, 20E feeding, and enzyme-linked immunosorbent assays of humoral 20E were performed by standard methods (SI Appendix).
Supplementary Material
Acknowledgments
We thank Michael O’Connor for Phantom enhancer DNA; Christen Mirth for advice regarding the 20E enzyme-linked immunosorbent assay and the consequences of EcR and Usp RNAi knock-down; Myriam Zecca for the UAS.dpp-GFP transgene; and Myriam Zecca, Andrew Tomlinson, and Rory Coleman and for discussion and critical comments on the manuscript. This work was supported by a Sir Henry Wellcome Postdoctoral Fellowship to J.P. and funding to G.S. from the Ellison Medical Foundation (AG-SS-2823-11), the Howard Hughes Medical Institute, and NIH grants R01 GM113000 and R35 GM127141.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018196117/-/DCSupplemental.
Data Availability.
All study data are included in the paper and SI Appendix.
References
- 1.Bryant P. J., Simpson P., Intrinsic and extrinsic control of growth in developing organs. Q. Rev. Biol. 59, 387–415 (1984). [DOI] [PubMed] [Google Scholar]
- 2.Day S. J., Lawrence P. A., Measuring dimensions: The regulation of size and shape. Development 127, 2977–2987 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Schwank G., Basler K., Regulation of organ growth by morphogen gradients. Cold Spring Harb. Perspect. Biol. 2, a001669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariharan I. K., Organ size control: Lessons from Drosophila. Dev. Cell 34, 255–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twitty V. C., Schwind J. L., The growth of eyes and limbs transplanted heteroplastically between two species of Amblystoma. J. Exp. Zool. Part A. Ecol. Genet. Physiol. 59, 61–86 (1931). [Google Scholar]
- 6.Silber S. J., Growth of baby kidneys transplanted into adults. Arch. Surg. 111, 75–77 (1976). [DOI] [PubMed] [Google Scholar]
- 7.Dittmer J. E., Goss R. J., Dinsmore C. E., The growth of infant hearts grafted to young and adult rats. Am. J. Anat. 141, 155–160 (1974). [DOI] [PubMed] [Google Scholar]
- 8.Felts W. J. L., Transplantation studies of factors in skeletal organogenesis. I. The subcutaneously implanted immature long-bone of the rat and mouse. Am. J. Phys. Anthropol. 17, 201–215 (1959). [DOI] [PubMed] [Google Scholar]
- 9.Bohn H., [Intercalary regeneration and segmental gradients in the extremities ofLeucophaea-larvae (Blattaria) : I. Femur and tibia]. Wilhelm Roux Arch. Entwickl. Mech. Org. 165, 303–341 (1970). [DOI] [PubMed] [Google Scholar]
- 10.Bryant P. J., Pattern formation in the imaginal wing disc of Drosophila melanogaster: Fate map, regeneration and duplication. J. Exp. Zool. 193, 49–77 (1975). [DOI] [PubMed] [Google Scholar]
- 11.Bryant S. V., French V., Bryant P. J., Distal regeneration and symmetry. Science 212, 993–1002 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Hariharan I. K., Serras F., Imaginal disc regeneration takes flight. Curr. Opin. Cell Biol. 48, 10–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., et al. , Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382, 133–138 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Zecca M., Basler K., Struhl G., Direct and long-range action of a wingless morphogen gradient. Cell 87, 833–844 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Nellen D., Burke R., Struhl G., Basler K., Direct and long-range action of a DPP morphogen gradient. Cell 85, 357–368 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Burke R., Basler K., Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development 122, 2261–2269 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Martín-Castellanos C., Edgar B. A., A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development 129, 1003–1013 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Chen C. M., Struhl G., Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126, 5441–5452 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Zecca M., Struhl G., Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development 134, 3001–3010 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Zecca M., Struhl G., Control of Drosophila wing growth by the vestigial quadrant enhancer. Development 134, 3011–3020 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Zecca M., Struhl G., A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 8, e1000386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmansa S., Hamaratoglu F., Affolter M., Caussinus E., Dpp spreading is required for medial but not for lateral wing disc growth. Nature 527, 317–322 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Bosch P. S., Ziukaite R., Alexandre C., Basler K., Vincent J.-P., Dpp controls growth and patterning in Drosophila wing precursors through distinct modes of action. eLife 6, e22546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J., et al. , Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, P1120–P1133 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh H., Irvine K. D., In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081–1088 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lander A. D., Pattern, growth, and control. Cell 144, 955–969 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubiger M., Palka J., Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev. Biol. 123, 145–153 (1987). [DOI] [PubMed] [Google Scholar]
- 28.Warren J. T., et al. , Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: Correlations with changes in gene activity. Dev. Dyn. 235, 315–326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant P. J., Levinson P., Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Dev. Biol. 107, 355–363 (1985). [DOI] [PubMed] [Google Scholar]
- 30.Bownes M., Dubendorfer A., Smith T., Ecdysteroids in adult males and females of drosophila-melanogaster. J. Insect Physiol. 30, 823–830 (1984). [Google Scholar]
- 31.Schwedes C. C., Carney G. E., Ecdysone signaling in adult Drosophila melanogaster. J. Insect Physiol. 58, 293–302 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Rogulja D., Irvine K. D., Regulation of cell proliferation by a morphogen gradient. Cell 123, 449–461 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Rogulja D., Rauskolb C., Irvine K. D., Morphogen control of wing growth through the Fat signaling pathway. Dev. Cell 15, 309–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence P. A., Struhl G., Casal J., Do the protocadherins Fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat. Cell Biol. 10, 1379–1382 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wartlick O., et al. , Dynamics of Dpp signaling and proliferation control. Science 331, 1154–1159 (2011). [DOI] [PubMed] [Google Scholar]
- 36.García-Bellido A. C., García-Bellido A., Cell proliferation in the attainment of constant sizes and shapes: The entelechia model. Int. J. Dev. Biol. 42, 353–362 (1998). [PubMed] [Google Scholar]
- 37.French V., Bryant P. J., Bryant S. V., Pattern regulation in epimorphic fields. Science 193, 969–981 (1976). [DOI] [PubMed] [Google Scholar]
- 38.Aegerter-Wilmsen T., Aegerter C. M., Hafen E., Basler K., Model for the regulation of size in the wing imaginal disc of Drosophila. Mech. Dev. 124, 318–326 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Eder D., Aegerter C., Basler K., Forces controlling organ growth and size. Mech. Dev. 144, 53–61 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Bryant P. J., Huettner B., Held L. I. Jr, Ryerse J., Szidonya J., Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev. Biol. 129, 541–554 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Gibson M. C., Schubiger G., Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs. Development 126, 1591–1599 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Aldaz S., Escudero L. M., Freeman M., Live imaging of Drosophila imaginal disc development. Proc. Natl. Acad. Sci. U.S.A. 107, 14217–14222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson P. R., Kirby K., Hilliker A. J., Phillips J. P., RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum. Mol. Genet. 14, 3397–3405 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Talamillo A., et al. , Smt3 is required for Drosophila melanogaster metamorphosis. Development 135, 1659–1668 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Coleman R. T., Struhl G., Causal role for inheritance of H3K27me3 in maintaining the OFF state of a DrosophilaHOX gene. Science 356, eaai8236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand A. H., Perrimon N., Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Lai S.-L., Lee T., Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 9, 703–709 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Basler K., Struhl G., Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368, 208–214 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Xu T., Rubin G. M., Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993). [DOI] [PubMed] [Google Scholar]
- 50.Capdevila J., Guerrero I., Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13, 4459–4468 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zecca M., Basler K., Struhl G., Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121, 2265–2278 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Tabata T., Kornberg T. B., Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76, 89–102 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Struhl G., Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Tabata T., Schwartz C., Gustavson E., Ali Z., Kornberg T. B., Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development 121, 3359–3369 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Giraldez A. J., Cohen S. M., Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development 130, 6533–6543 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Baena-López L. A., Franch-Marro X., Vincent J.-P., Wingless promotes proliferative growth in a gradient-independent manner. Sci. Signal. 2, ra60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexandre C., Baena-Lopez A., Vincent J.-P., Patterning and growth control by membrane-tethered Wingless. Nature 505, 180–185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang J., Wu S., Barrera J., Matthews K., Pan D., The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421–434 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Neumann C. J., Cohen S. M., Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122, 1781–1789 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Cho E., Irvine K. D., Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131, 4489–4500 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Spencer F. A., Hoffmann F. M., Gelbart W. M., Decapentaplegic: A gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28, 451–461 (1982). [DOI] [PubMed] [Google Scholar]
- 62.Schubiger M., Truman J. W., The RXR ortholog USP suppresses early metamorphic processes in Drosophila in the absence of ecdysteroids. Development 127, 1151–1159 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Schubiger M., Carré C., Antoniewski C., Truman J. W., Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development 132, 5239–5248 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Cherbas L., Hu X., Zhimulev I., Belyaeva E., Cherbas P., EcR isoforms in Drosophila: Testing tissue-specific requirements by targeted blockade and rescue. Development 130, 271–284 (2003). [DOI] [PubMed] [Google Scholar]
- 65.McGuire S. E., Mao Z., Davis R. L., Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Doherty D., Feger G., Younger-Shepherd S., Jan L. Y., Jan Y. N., Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10, 421–434 (1996). [DOI] [PubMed] [Google Scholar]
- 67.Champlin D. T., Truman J. W., Ecdysteroids govern two phases of eye development during metamorphosis of the moth, Manduca sexta. Development 125, 2009–2018 (1998). [DOI] [PubMed] [Google Scholar]
- 68.Nijhout H. F., et al. , The control of growth and differentiation of the wing imaginal disks of Manduca sexta. Dev. Biol. 302, 569–576 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Nijhout H. F., Grunert L. W., The cellular and physiological mechanism of wing-body scaling in Manduca sexta. Science 330, 1693–1695 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Andersen D. S., Colombani J., Léopold P., Coordination of organ growth: Principles and outstanding questions from the world of insects. Trends Cell Biol. 23, 336–344 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Herboso L., et al. , Ecdysone promotes growth of imaginal discs through the regulation of Thor in D. melanogaster. Sci. Rep. 5, 12383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hadorn E., Regulation and differentiation within field-districts in imaginal discs of Drosophila. J. Embryol. Exp. Morphol. 1, 212–216 (1953). [Google Scholar]
- 73.Nöthiger R., Schubiger G., Developmental behaviour of fragments of symmetrical and asymmetrical imaginal discs of Drosophila Melanogaster (Diptera). J. Embryol. Exp. Morphol. 16, 355–368 (1966). [PubMed] [Google Scholar]
- 74.Bryant P. J., Fraser S. E., Wound healing, cell communication, and DNA synthesis during imaginal disc regeneration in Drosophila. Dev. Biol. 127, 197–208 (1988). [DOI] [PubMed] [Google Scholar]
- 75.Jursnich V. A., Fraser S. E., Held L. I. Jr, Ryerse J., Bryant P. J., Defective gap-junctional communication associated with imaginal disc overgrowth and degeneration caused by mutations of the dco gene in Drosophila. Dev. Biol. 140, 413–429 (1990). [DOI] [PubMed] [Google Scholar]
- 76.Lecuit T., Cohen S. M., Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development 125, 4901–4907 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Vuilleumier R., et al. , Control of Dpp morphogen signalling by a secreted feedback regulator. Nat. Cell Biol. 12, 611–617 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Cadigan K. M., Fish M. P., Rulifson E. J., Nusse R., Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell 93, 767–777 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Fujise M., et al. , Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130, 1515–1522 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Han C., Yan D., Belenkaya T. Y., Lin X., Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132, 667–679 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Colombani J., Andersen D. S., Léopold P., Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582–585 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Garelli A., Gontijo A. M., Miguela V., Caparros E., Dominguez M., Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Vallejo D. M., Juarez-Carreño S., Bolivar J., Morante J., Dominguez M., A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science 350, aac6767 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Garelli A., et al. , Dilp8 requires the neuronal relaxin receptor Lgr3 to couple growth to developmental timing. Nat. Commun. 6, 8732 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Y., Qiu Y., Chen W., Nie Q., Lander A. D., Scaling a dpp morphogen gradient through feedback control of receptors and Co-receptors. Dev. Cell 53, 724–739.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelnar C., Savage M., Saenger P., Cowell C., Growth Disorders (Hodder Arnold, ed. 2, 2007). [Google Scholar]
- 87.Funakoshi Y., Minami M., Tabata T., Mtv shapes the activity gradient of the Dpp morphogen through regulation of thickveins. Development 128, 67–74 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the paper and SI Appendix.