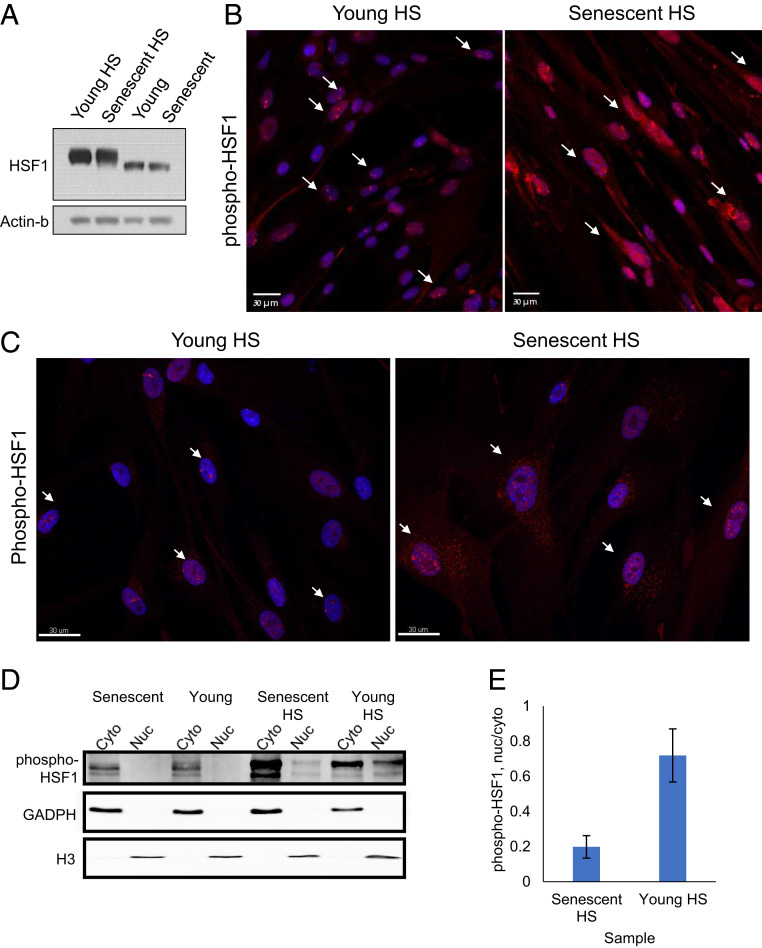
Fig. 3.
HS-mediated nuclear localization and distribution of HSF1 are hampered in senescent cells. (A) The total levels of HSF1 in young and senescent cells are very similar (SI Appendix, Fig. S4 A–C). (B) Immunofluorescence staining of phospho-HSF1 in young and senescent HS cells show increased cytoplasmic staining in senescent cells. Additionally, while most young cells show 1–4 single bright nuclear foci of phospho-HSF1 upon HS, most senescent cells show many disorganized foci of phospho-HSF1. The white arrows show examples of cells with distinct 1–4 foci in young cells (Left) and disorganized HSF1 localization in old cells (Right). (Scale bar, 30 μm). (C) Confocal 3D imaging of phospho-HSF1 in young and senescent HS cells revealed a closer look of the nuclear foci distribution in young cells and its impairment in senescent cells. The white arrows show examples of cells with distinct 1–4 foci in young cells (Left) and disorganized HSF1 localization in old cells (Right). Additional images and the quantification of the number of foci is shown in SI Appendix, Fig. S4 E–G. (Scale bar, 30 μm). (D) A representative WB of nucleus–cytoplasm fractionated cells. Phospho-HSF1 is absent from cell nuclei before HS. Following HS in young cells, nuclear phospho-HSF1 is increased, whereas in senescent cells, the fraction of nuclear phospho-HSF1 is significantly lower. GAPDH and histone H3 present cytoplasmic or nuclear enrichment, respectively. (E) Quantification of WB densitometry for phospho-HSF1 using Fiji. Phospho-HSF1 was quantified in each fraction, and the nucleus was divided by the corresponding cytoplasmic fraction. The bars represent the mean and SE of three biological replicates.